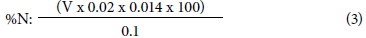Introduction
Coffee is one of the most internationally traded food products and the second most consumed beverage in the world behind water. Its contribution to the socioeconomic development of tropical countries is a key factor with about 70% of the world production of coffee beans produced by small farmers (Fridell et al., 2008). In Peru, the coffee crop occupies almost 350,000 ha and generates employment for 2 million growers (Junta Nacional del Café, 2020). But one of the most important threats to coffee production is climate change which will increase temperatures by an average of 4.8°C by the year 2100 (DaMatta et al., 2018). It is anticipated that rising temperatures will be coupled with variations in precipitation patterns, intensifying drought conditions in many agricultural areas. Also, the occurrence of extreme weather events and fluctuations in the distribution of rainfall in both time and space are contributing factors to alterations in soil moisture levels, leading to an increase in saline soils (Corwin, 2021). According to Corso et al. (2020), drought causes a decrease or cessation of cell expansion, leading to a delay in plant growth. In plants, this produces wilting, chlorosis (yellowing of leaves), and changes in the number of leaves and leaf area. Additionally, elevated temperatures may increase the respiratory rate, disrupt the synthesis of chlorophyll, decrease net photosynthesis, and stimulate the synthesis of reactive oxygen species (Sharkey & Schrader, 2006; Wang et al., 2018; Mittler et al., 2022).
Likewise, during previous decades research focused on limiting the detrimental effects of coffee leaf rust, caused by Hemileia vastatrix L., a critical pathogen in coffee. For that reason, breeders have produced plants with resistance to this disease (Zambolim, 2016). However, low attendance has been addressed to produce plants with tolerance or resistance to the harsh conditions caused by climate change that may become increasingly problematic in the future. Despite the effects of heat and drought on plants that have been widely studied for important crops (Borjas et al., 2020; Handayani & Watanabe, 2020; Alsamir et al., 2021), information on coffee is still restricted, particularly to new cultivars in Perú, such as Ouro Verde IAC H 5010-5. This cultivar is susceptible to coffee leaf rust but has high vigor, productivity, and organoleptic quality (Fazuoli et al., 2000).
It is important to study the combined effect of stresses to quantify and document the physio-morphological response of the seedlings of coffee cv. Ouro Verde IAC H 5010-5 to low availability of water and elevated temperature, since many scientific reports have proved that the responses of plants to single and combined stress are different. Both elevated temperatures and drought have a negative impact on coffee production. Subsequently, these factors force small farmers to migrate to other suitable areas for the cultivation of this crop (Hussain et al., 2021) and alter the morphology, physiology, biochemistry, and yield of tropical crops (Borjas et al., 2019). The severity of impact of elevated temperature and drought on crops depends on weather conditions, crop management, levels of stress, and genetic material (Ullah et al., 2019; Reshma et al., 2021; Yang et al., 2021). Consequently, it is convenient and necessary to conduct future studies on different cultivars to increase our knowledge of their responses to climate change.
Materials and methods
Plant material used was the coffee cultivar Ouro Verde IAC H 5010-5, obtained from the Peruvian Coffee Gene bank located in San Ramón (province of Chanchamayo, department of Junín). This assessment was carried out at the Universidad Nacional Agraria La Molina (Lima, Peru) (12°04'55" S; 76°56'53" O) from December 2021 to May 2022.
Once harvested, mature fruits were pulped manually to avoid damage to the seeds. After that, fruits were fermented for 24 h to facilitate mucilage removal. The bean parchment was discarded. To stimulate germination, the seeds were soaked in water for 24 h. They were then sowed in seed trays under a controlled environment that maintained the optimal temperature for growth. Once the seedlings reached the appropriate stage after 50 d, they were transferred to 1 L polyethylene bags, 25.4 cm high and 12.7 cm wide. The substrate used was composed of Sphagnum peat moss and 15% perlite.
Adequate seedling nutrition was guaranteed by supplying 100 ml of solution prepared by combining 2 ml of micronutrients and 5 ml of macronutrients per liter of "Solución nutritiva hidropónica La Molina®" (Fundación para el Desarrollo Agrario - FDA) nutrient solution. The fertilization started 78 d after sowing and was done once a week for six weeks.
Two levels of water availability and temperature were studied in this work. For this purpose, plants were grown for three months with a water input in weight (g) corresponding to the amount of water present at 0.3 bar in the substrate, representing 100% available water. After that period the plants were subjected to different treatments with weekly evaluations for the following seven weeks. Water availability had two levels: well-watered (100% available water) (W) and drought stress (< 50% available water) (-W), and the temperatures examined were air temperature: 22.7°C (T) and elevated temperature (~2.5°C above air temperature) (+T). The combination of the levels resulted in four treatments with 14 replicates, one plant per experimental unit (Tab. 1).
To determine the volume of water needed to reach 50% of available water, we followed Wang et al. (2017). First, it was necessary to calculate the percentage of available water in weight (%) at 15 and 0.3 bars, representing the two moisture levels that determine the available water, as well as the bulk density of the substrate. With these data, we calculated the approximate dry weight of the substrate in the bags, to which a calculated amount of water was added to maintain a state of water stress below 50% of available water. To determine the reference weight for this stress treatment, the average total weight of the bag with substrate and water was considered under stress conditions. Subsequently, the bags subjected to this treatment were weighed weekly to ensure that they did not exceed the predetermined stress state weight.
To increase the temperature, a 1 m-tall tunnel was designed using PLASTERMIC DH2A (SOTRAFA S.A.), a transparent agricultural plastic film that allows light to enter. The coffee seedlings grew into this tunnel. Furthermore, the temperature into and out of the tunnel was recorded using a datalogger Elitech RC-4HC (Elitech UK Ltd, UK).
Growth
After seven weeks of treatment, the number of leaves was recorded. Height (cm) was measured using tape from the stem base to the apical bud, and for stem diameter (mm) measuring, a vernier caliper was used at the stem base. Root length (cm), and leaf area (cm2) for each seedling were quantified, employing ImageJ software. Dry biomass was measured. To obtain the dry weight of the shoot and roots, the coffee seedlings were placed in an oven at 70°C for 2 d.
Growth indices
With the information from the evaluation of growth, some indices such as the sturdiness index (SI) (Enquist & Niklas, 2002), Dickson index (DI) (Dickson, 1960), and dry shoot: root dry biomass ratio (Sd/Rd), were calculated.
The following equations were used:
where:
TDB = is total dry biomass (g);
SI = sturdiness index (cm/mm);
Sd = shoot dry biomass;
Rd = root dry biomass (g).
Content of total nitrogen
At the Soil Laboratory "Sven Villagarcía Hermosa" - Universidad Nacional Agraria La Molina, we used the micro Kjeldahl method (Bazan, 1996). The total leaves were collected for this analysis. Foliar material was dried on a stove at 70°C for 24 h and later ground for an easier digestion process. From the processed leaf tissue, 0.1 g per sample was taken that was digested with K2SO4, CuSO4, and H2SO4. The resulting product was subjected to distillation in the presence of 50% NaOH and collected in 2% H3BO3. This distillate was titrated with H2SO4 at 0.02 N. This acid expenditure (V) was entered into the equation to obtain the percentage of foliar nitrogen.
Photosynthesis and water use
The photosynthetic performance of coffee under high temperatures and drought was examined with the CIRAS-3 DC CO2/H2O gas analyzer that was used to evaluate the content of CO2 in the intercellular space of the leaves (Ci, μmol mol-1), net CO2 assimilation rate (A, μmol CO2 m-2 s-1), stomatal conductance (gs, mmol H2 O m-2 s-1), transpiration rate (E, mmol H2O m-2 s-1), vapor pressure deficit (VPD, KPa), and water use efficiency (WUE, mmol CO2 mol-1 H2 O). The measurements were made in mature leaves (from the middle part of the plants) at 71 d (first sampling), 78 d (second sampling), 85 d (third sampling), and 93 d (fourth sampling) after transplanting. All assessments were taken in the morning between 9-11 a.m.
Climate data
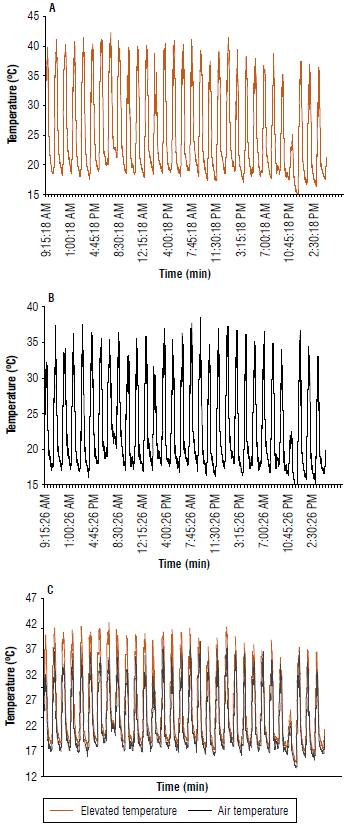
Temperature variation was recorded every 15 min throughout the day during the experiment. Air temperature (T) varied from 13.7°C to 38.5°C and had an average of 22.7°C. The treatments with elevated temperature (+T) varied from 14.7°C to 42.3°C with a mean temperature of 25.2°C (Fig. 1).
Results and discussion
To deepen the knowledge of the impact of isolated and combined drought and heat, we studied the effects of two levels of both water (100% available water [W] and < 50% of available water [-W]) and temperature (air temperature [T] and elevated temperature ~2.5°C above air temperature [+T]) on the growth, the content of nitrogen, and photosynthesis of coffee seedlings cv. IAC H 5010-5.
During the experiment growth was disrupted by the [-W T] and [-W +T] treatments, both decreasing the height and diameter of the shoot, root length, and the number of leaves per plant (NL) (P≤0.05) (Figs. 2-3). Additionally, the effect of water scarcity was more prominent than the increased temperature for plant height, root length, and NL.
Drought is considered the most adverse environmental factor that modifies plant growth (Seleiman et al., 2021) by altering its physiology, biochemistry, and chemical composition and the allocation of photosynthetic products (Wang et al., 2018; Hussain et al., 2019; Borjas et al., 2020; Schönbeck et al., 2021). Growth reduction is the first visual indicator that plants are under drought stress, as informed in previous experiments with corn (Van Nguyen et al., 2022), soybean (Imran et al., 2021), and coffee cvs. IAPAR 11260, IPR 100 and IPR 103 (Carvalho et al, 2017). However, there are few studies on the cultivar IAC H 5010-5.
The plants subjected to the treatments [-W T] and [-W +T] had a lower leaf area and shoot dry biomass than the other ones (P<0.05) (Figs. 4-5). While both [W +T] and [-W +T] plants caused a reduction in the root dry weight. For this latter variable, temperature acted individually, suggesting that the effects of high temperature on plant development are water-dependent (Fahad et al., 2017). Indeed, plants well-watered and under high temperatures can allocate more photosynthetic products toward the aerial part than the roots (Shinohara & Leskovar, 2014; Reddy et al., 2017) as observed in this study, where plants under [W +T] showed high shoot dry weight but low shoot dry biomass (P≤0.05). This result can be related to the fact that the same treatment increased the shoot dry: root dry biomass ratio (P≤0.05) (Fig. 6).
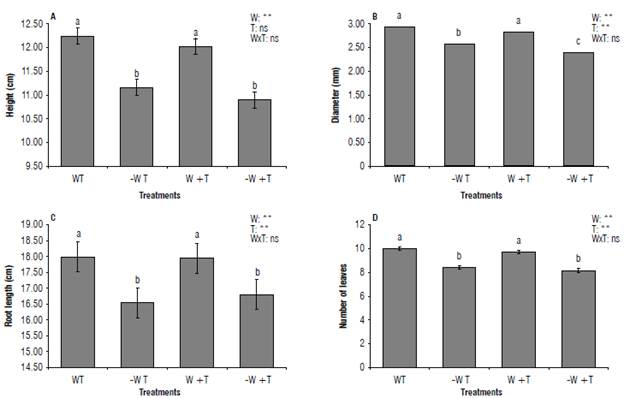
FIGURE 2 Growth of coffee seedlings cv. IAC H 5010-5 subjected to levels of water and temperature. A) Plant height; B) stem diameter; C) root length; D) number of leaves. W: 100% available water; -W: < 50% of available water; T: air temperature (22.7°C); +T: elevated air temperature (22.7 + 2.5°C). Different letters indicate statistical differences, and error bars represent the standard error values (Scott & Knott test at 95%).
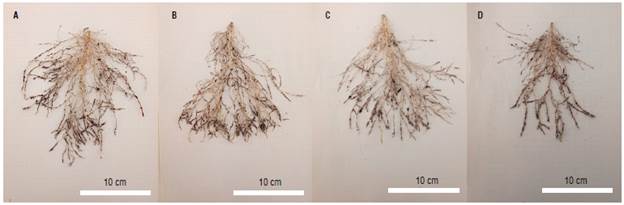
FIGURE 3 Coffee cv. Ouro Verde IAC H 5010-5 roots subjected to treatments: A) WT; B) -WT; C) W+T; D) -W+T, where W: 100% available water; -W: < 50% of available water; T: air temperature (22.7°C); +T: elevated air temperature (22.7 + 2.5°C).
Conversely, when the high temperature is accompanied by water scarcity, both can be extremely harmful since they can disrupt the adequate physiology of plants leading them to decrease biomass production as recorded in this assessment where shoot and root dry biomass reduced in plants subjected to [-W +T] (P≤0.05) (Fig. 5) (Raja et al., 2020). Our results agree with Shinohara and Leskovar (2014), and Zhou et al. (2017) for Cynara cardunculus L. var. scolymus (L.) Fiori and Solanum lycopersicum. The changes in morphological traits caused by [-W +T] increased the sturdiness index (SI). High values of SI suggest that coffee seedlings are less able to resist abiotic stress conditions.

FIGURE 4 Coffee cv. Ouro Verde IAC H 5010-5 shoot subjected to treatments: A) WT; B) -WT; C) W+T; D) -W+T, where W: 100% available water; -W: < 50% of available water; T: air temperature (22.7°C); +T: elevated air temperature (22.7 + 2.5°C).
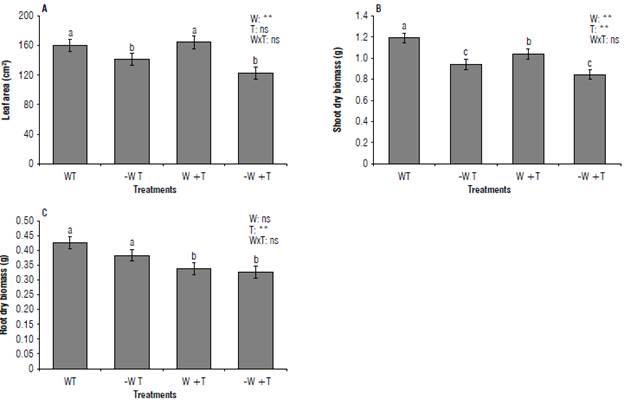
FIGURE 5 Shoot and root growth parameters of coffee seedlings cv. IAC H 5010-5. A) Leaf area; B) dry biomass of the shoot; C) dry biomass of the roots. W: 100% available water; -W: < 50% of available water; T: air temperature (22.7°C); +T: elevated air temperature (22.7 + 2.5°C). Different letters indicate statistical differences, and error bars represent the standard error values according to the Scott & Knott test (95%).
Likewise, [-W +T] decreased the Dickson quality index that confirmed those plants under both stresses have not adapted to tolerate harsh conditions in the field (Lin et al., 2019) (P<0.05) (Fig. 6).
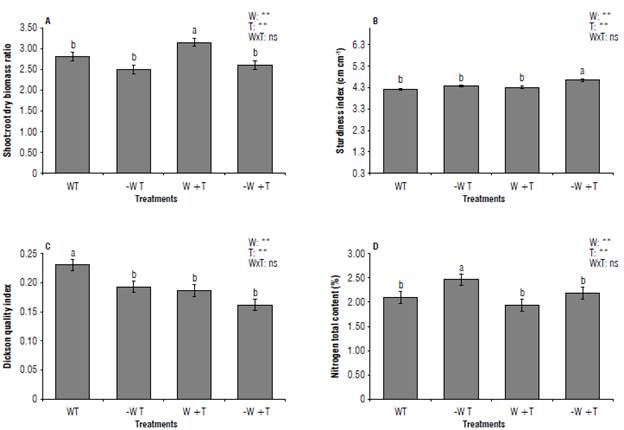
FIGURE 6 Variables measured in coffee seedlings cv IAC H 5010-5. A) Shoot dry: root dry biomass ratio; B) sturdiness index; C) Dickson quality index; D) total nitrogen content. W: 100% available water; -W: < 50% of available water; T: air temperature (22.7°C); +T: elevated air temperature (22.7 + 2.5°C). Different letters indicate statistical differences and error bars represent the standard error values according to the Scott & Knott test (95%).
On the other hand, nitrogen (N) is an essential nutrient related to plant development and productivity (Hessini et al, 2019; Tadesse et al, 2019; Xiong et al, 2021). The availability of N in the soil is subject to climatic conditions, particularly, the levels of water in the soil. This means that low levels of water in the soil can limit N uptake by plants (Liu et al, 2013; He & Dijkstra, 2014; Borjas et al, 2019). Nevertheless, an unexpected result was noticed since the highest content of N (%) was recorded in the plants exposed to [-W T] with 2.46 compared to the 1.9 for [W +T] treatment (Fig. 6).
The quantity of N in plants under the drought treatments can be explained by the activation of the genes responsible for the uptake and assimilation of nitrogen (AMT1;3, AMTl;lb, NRT1;2 and NRT2;5, NR, GS2, and GSI;2), that enhance the absorption of N as reported in maize where drought stress increased the level of N in mature leaf and roots (Wang et al, 2017). Also, a higher percentage of total nitrogen is most likely a result of the accumulation of nitrate in the leaves that is brought about by the impact of stress that had a greater effect on assimilation as opposed to the transport and/or absorption of nitrogen (Martinez et al, 2020).
Photosynthesis is defined as the use of sunlight to synthesize carbohydrates to meet the needs of plants. The evaluation of photosynthesis in this research included the quantification of net C02 assimilation rate (A), the C02 content in the intercellular space of the leaves (Ci), stomatal conductance (gs), transpiration rate (E), vapor pressure deficit (VPD), and the use of water use efficiency (WUE) (Fig. 7).
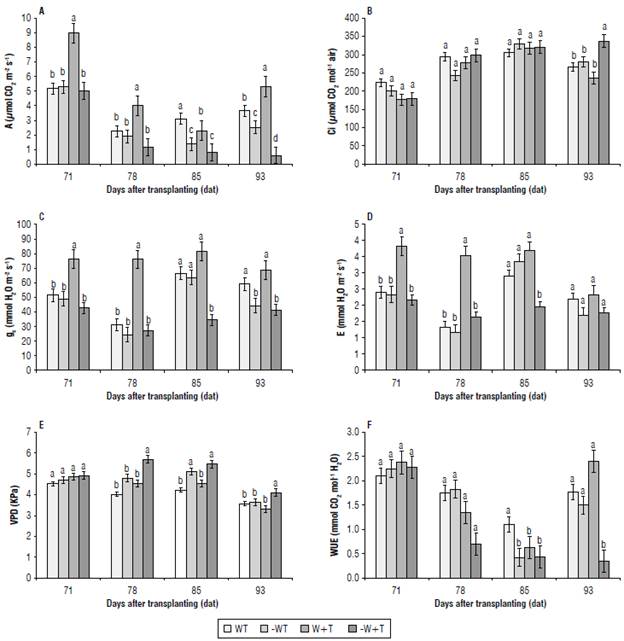
FIGURE 7 The variable measured in coffee seedlings cv. IAC H 5010-5. A) A: net C02 assimilation rate; B) Ci: content of C02 in the intercellular space of the leaves; C) gs: stomatal conductance; D) E: transpiration rate; E) VPD: vapor pressure deficit; F) WUE: water use efficiency. W: 100% available water; -W: < 50% of available water; T: air temperature (22.7°C); +T: elevated air temperature (22.7 + 2.5°C). Different letters indicate statistical differences and error bars represent the standard error values according to Scott & Knott test (95%).
Drought can have a detrimental effect on cell turgidity, decreasing the photosynthetic area, while stomatal closure leads to decreasing gas exchange limiting the functioning of the photosynthetic apparatus (Semedo et al, 2018; Helm et al, 2020). Similar effects were reported by Rodrigues et al. (2018) in coffee subjected to high temperatures.
Our research demonstrated the modulator effect of the water levels in soil on plant responses to high temperatures (Ayub et al, 2021). In fact, [W +T] increased A and gs suggesting that with high water availability C3 plants, particularly coffee, can have their growth enhanced. In contrast, the combination of high temperatures and water scarcity [-W +T] decreased A, gs, E, and WUE (P≤0.05) at 85 and 93 d after transplanting (third and fourth sampling dates). This response was also reported by Hussain et al. (2019) and Raja et al. (2020) in maize (C4) and tomato (C3). The fall of carbon net assimilation, caused by the reduction in the carboxylation activity of RuBisCo (Souza et al, 2020; Almeida et al, 2021; León et al, 2022), increased the quantity of carbon intracellular and the VDP (P≤0.05) (Fig. 7).
Conclusion
Assessing the morphology, nitrogen content, and physiology of coffee seedlings cv. IAC H 5010-5 under increased temperature and water scarcity demonstrated that the isolated and combined occurrence of these stresses produced differentiated responses that were more prominent than the effects of combined stresses. In general, the impact of the less than 50% available water was more predominant than increased temperatures in reducing plant growth and physiology. Likewise, the effect of temperature was water-dependent. Furthermore, coffee seedlings under drought showed high nitrogen content suggesting that this cultivar possesses physiological strategies to face the low availability of water in the soil.