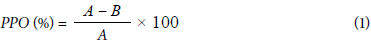Introduction
Enzymatic browning is one of the problems that occurs during the processing and storage of some fruit juices such as apple and banana juices (Moon et al., 2020). Generally, browning causes changes in loss of nutritional, functional, and sensory values as a result of darkening; and it leads to lowered quality of fruit juices. The browning phenomenon is an enzymatic process that occurs in juice of some fruits due to polyphenol oxidase (PPO) activity (enzyme commission-EC: 1.14.18.1) (Jia et al, 2023). PPO refers to a copper containing enzyme that catalyzes in the presence of oxygen with the hydroxylation of monophenols to diphenols and oxidation of dihydroxyphenols to quinone that are then subjected to more reactions, leading to a polymerizing pigment (Yildiz et al., 2022). Quince (Cydonia oblonga Mill.) fruit has been used in juice processing in recent years (Llupa et al., 2022). The quality of the quince juice is influenced by color-related changes during processing and storage. Because PPO is naturally found in quince fruit, the enzyme produces browning pigments through oxidation of phenolic compounds. So, inhibition of the PPO activity is very important. There are many synthetic substances used to inhibit PPO enzyme that eliminate fruit browning and their juices: sulphites (Muñoz-Pina et al., 2022), sodium chloride (Alipoorfard et al., 2020), and ascorbic acid (Xu et al, 2022). However, these have negative effects on human health and have increased restrictions when used as browning inhibitors (Arnold & Gramza-Michatowska, 2022). Thus, a demand of consumers for substituting these antibrowning compounds with natural compounds has increased (Jirasuteeruk & Theerakulkait, 2020). The inhibitory effect of various natural agents on PPO and the degree of browning have been reported: these include onion extract (Bernas & Jaworska, 2021), honey extract (de la Rosa et al., 2011), green tea extract (Klimczak & Gliszczynska-Swiglo, 2017), shallot extract (Phaiphan et al., 2019), and galla rhois (Lee et al., 2022). The role and effectiveness of plant peel extracts depends on the polyphenol oxidase (PPO) source, the food matrix and the method and condition of the extraction. One of the apple juice processing wastes includes peels that contain numerous phenolic compounds. These compounds were identified as hydroxycinnamic acids, quercetin, catechin, and phloridzin (Bitalebi et al., 2019; Ahmad et al., 2020). The mechanism of reduced browning includes, probably, the action of these compounds as competitive inhibitors for PPO that have similar structures with enzyme substrates (Bobo et al., 2022). The aim of the present study was to compare the inhibitory action of alcoholic apple peel extract with aqueous extracts and their concentrations on PPO activity and browning in quince juices during cold storage.
Materials and methods
Raw plant material, chemicals, and reagents
A local variety of quince fruit (Cydonia oblonga Miller) and apple fruit (cv. Golden Delicious) were obtained from a supermarket in Mosul city, Iraq and stored in the refrigerator after being rinsed with water before being used in the experiments. Catechol, L-ascorbic acid, gallic acid were purchased from Sigma- Aldrich Chemical Co. Ltd (St. Louis, MO, USA). Folin-Ciocalteu reagent were obtained from Merck (Darmstadt, Germany). The common chemicals such as sodium hydroxide, ethyl alcohol (95%), as well as sodium carbonate were of analytical reagent grade, and solutions were prepared using distilled water.
Preparation of alcoholic and aqueous apple peel extract powders
The preparation was done following the procedure reported by Thanoun and Al-Jammaas (2022) with some modifications. Ripe apple fruits were washed with water; fruit peels were then manually removed and dried at 40°C until reaching a constant weight. An electrical mill was used to ground dried apple peels into fine powder. The powder was stored in a freezer at -18°C before extraction. The amount of apple peel powder was divided into two groups: the first group was extracted by 80% ethanol as alcoholic extraction for 72 h with shaking (mixing ratio 1:10 w/v). Afterwards, the obtained extract was concentrated by using a vacuum evaporator to remove ethanol by centrifuging for 15 min at 4°C and 5,000 rpm .The extract supernatant was dried in a lyophilizer and stored at -80°C until required as ethanolic apple peel extract (EAPE). The second group was extracted with distilled water as aqueous extraction, shaking for 72 h (1:20 w/v as mixing ratio). The aqueous extract was then centrifuged, dried as above, and used as aqueous apple peel extract (AAPE). For alcoholic and aqueous apple peel extracts, the percentages of extract yield were 4.64 and 1.56 g/100 g apple fruit, respectively.
Preparation of quince juice and its treatments
Hand peeled fruits were crushed using a blender (Waring commercial blender). After that, the crushed quince was filtered through two layers of muslin cloth. The quince juice samples were then divided into four groups: two of them were treated with alcoholic apple peel extract, and the other two were treated with aqueous apple peel extract. For each type of extract 0.3% and 3% concentrations were used as added concentrations in the quince juice. The control sample was quince juice without extract.
Analyses
Polyphenol oxidase (PPO) was extracted from the quince juice; its activity assay was based on the method of de la Rosa et al. (2011) with some modifications: firstly, the extraction of the enzyme was accomplished by mixing the same volumes of juice and phosphate buffer (0.05 M, pH=7), at 4°C for 60 min (obtained by using iced water bath) and then centrifuged at 500 rpm at 4°C for 10 min and filtered. The supernatant (enzyme extract) was used for PPO activity by mixing 0.5 ml of enzyme extract with 1 ml of catechol solution 0.1 M and 2 ml of phosphate buffer solution (0.05 M, pH 7.2). The absorbance values were recorded with a spectrophotometer at 420 nm every 30 s for 3 min at 30°C as incubation temperature. The activity of the PPO enzyme was obtained based on the slope of the linear portion of the curve plotted among absorbance values at 420 nm against time. One unit of enzyme activity was expressed as the change in absorbance at 420 nm per min per ml under the assay conditions. The percentage of polyphenol oxidase activity inhibition (PPO) was calculated using the following equation:
where:
A = The enzyme activity value for quince juice sample without added extracts.
В = The enzyme activity value for quince juice sample with added extracts.
The browning index of quince juice samples was estimated at 420 nm with a UV-VIS spectrophotometer (Iqbal et al, 2018; Iqbal et al., 2020). The percentage of browning inhibition (BI) was computed using the following equation:
where:
A = The absorbance value at 420 nm for quince juice sample without added extracts.
В = The absorbance value at 420 nm for quince juice sample with added extracts.
Total phenolic content (TPC) of quince juice was measured according to the Folin-Ciocalteu reagent procedure reported by Trigo et al. (2020) with some modifications: 0.5 ml of quince juice ml was added to a test tube containing 0.5 ml of Folin-Ciocalteu reagent and after 4 min, 2 ml of Na2C03 solution with 20% w/v was added. The total volume was adjusted to 5 ml with distilled water. The tubes were left for 120 min at room temperature in the dark. The absorbance value was analyzed at 760 nm in a UV-VIS spectrophotometer, and the results were expressed in mg gallic acid equivalents/100 ml through a gallic acid calibration curve. Total soluble solids (TSS) were determined as degrees Brix by using an Abbe refractometer (Atago, Tokyo, Japan). The pH values of the quince juice samples were determined using a WTW pH-meter at 25°C (Iqbal et al., 2020). Titratable acidity content was performed as malic acid (%) after titration with sodium hydroxide standard solution (0.1 N) in the presence of the colored indicator (Saberi et al, 2019).
Statistical analysis
A factorial completely randomized experimental design was conducted in triplicate (repeated three times) for research experiments. The two-way analysis of variance (ANOVA) was carried out to test the effects of peel extract method, peel extract concentration, storage time, and their interactions. Duncan's test was used to compare the means using the SAS program (Proc. GLM, SAS program, version 9.3, SAS, 2012). The differences atP<0.05 were considered statistically significant.
Results and discussion
Effect of apple peel extract addition on PPO enzyme inhibition in quince juice during storage
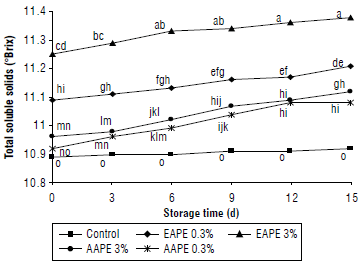
The inhibition of PPO activity (%) in quince juice treated with both ethanolic (EAPE) and aqueous (AAPE) apple peel extracts and stored for 15 d at 4°C is presented in Figure 1. The concentrations of each added extract were 0.3% and 3%. The enzyme inhibition percent differed according to the extract method, concentration added to the extract, and the storage time. The result showed that inactivation percent of PPO in the EAPE treated quince juice with 3% as concentration extract was a larger significant (P<0.05) compared to 0.3% treated juice samples.
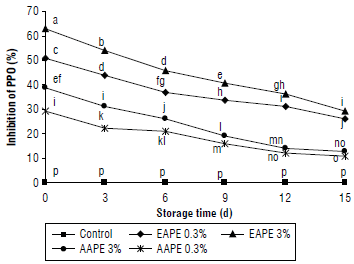
FIGURE 1 Effect of addition of alcoholic apple peel extracts to quince juice on inhibition percent of polyphenol oxidase (PPO). The same letters indicated no significant differences according to the Duncan test (P>0.05).
Based on 12th and 15th d of storage, the percent of inhibition of PPO enzyme in the 3% AAPE treated juice samples was insignificantly greater than juice samples treated with concentration of 0.3%. Quince juice samples treated with both concentrations of aqueous apple peel extract had significantly (P<0.05) lower PPO inhibition percentages (29% and 39%) than samples treated with same concentrations of alcoholic apple peel extract (51% and 63%) on 0 day of storage at 4°C.
According to Figure 1, the highest percent of inhibition of quince juice PPO enzyme was obtained with an alcoholic extract of apple peel, and this bioactivity could be related to its high content of phenolic compounds (Machado et al., 2013). These results strongly supported the addition of the alcoholic, aqueous of natural extracts for effectiveness in inhibiting the juice PPO enzyme. In general, our results agree with other authors (Klimczak & Gliszczynska-Swiglo, 2017; Jirasuteeruk & Theerakulkait, 2020; Lee et al., 2022) who studied the effects of shallot extract, mango peel extract, and galla rhois water extract on polyphenol oxidase activity for apple juice, potato puree, and apple juice.
Effect of apple peel extract supplementation on browning index inhibition in quince juice during cold storage
Changes in browning of quince juice in terms of browning index (BI) values are shown in Figure 2. Addition of extracts of apple peel with different concentrations caused significant variations in the BI values of quince juice. The treatment of quince juice with 3% alcoholic extract of apple peel showed more inhibitory effect on BI values of juice than that of the 0.3% of the same extract at the end of the storage. The BI (%) of water extracts of apple peel added juice samples was significantly decreased with the decrease of storage day (P<0.05): it was 15% and 9% on the 15th d of cold storage as compared to 0 d (34% and 28%) for extract concentrations of 3% and 0.3%, respectively. Results indicated that the browning (in terms of inhibition percentage of BI) of quince juice after being supplemented with alcoholic extracts had higher values in comparison with aqueous extracts (Fig. 2).
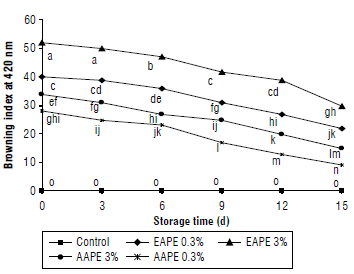
FIGURE 2 Effect of the addition of alcoholic apple peel extracts to quince juice on inhibition percent of browning index (BI). The same letters indicated no significant differences according to the Duncan test (P>0.05).
Samples treated with 3% EAPE showed the highest inhibition percentage of BI values of 52%, followed by 0.3% EAPE (40%), 3% AAPE (34%) and 0.3% AAPE (28%). Regardless of the natural anti-browning agent used, the findings described above are similar to that obtained by others (Altunkaya & Gökmen, 2008; Jirasuteeruk & Theerakulkait, 2020; Shomodder et al., 2021).
Effect of supplementation of apple peel extract on phenolic content in quince juice during storage at 4°C
The results of the total phenolic contents (mg/100 ml) in quince juice treated with apple extracts during storage are shown in Figure 3. The thermally pasteurized quince juice contained 49.18 mg/100 ml phenolics, but this amount significantly decreased by 2.62% on the 15th d of storage at 4°C. Treatment of quince juice with EAPE and AAPE revealed significant differences compared to untreated juice samples at 0 and 15th d of storage. TPCs were increased throughout storage time. The higher added concentrations of extract had significantly more TPC than the lower concentrations. Due to the high content of extracted phenolics using the alcoholic method, the values of TPC in alcoholic extracts treated juices were found to be larger (53.05 and 54.63 mg/100 ml), compared to juice samples treated with aqueous extracts (50.43 and 51.74 mg/100 ml), at the end of the storage period (Fig. 3).
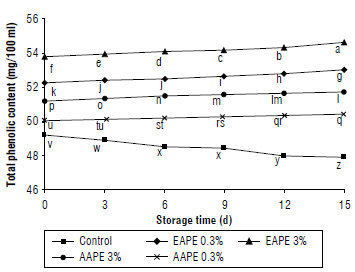
FIGURE 3 Effect of the addition of alcoholic apple peel extracts to quince juice on total phenolic content (mg/100 ml). The same letters indicated no significant differences according to the Duncan test (P>0.05).
Data for the effect of storage time on the amount of total phenolics in the juices showed significant increases in TPC with increased storage (15 d vs. 0 d). The results of TPC are in accordance with Altunkaya et al. (2013) who found significantly increases in the phenolic content of apple juice supplemented with pomegranate peel extracts. The results of this study are similar to other studies that showed an increase of phenolic values with an increment of natural extract concentrations in the fresh cut potato slices (Liu et al., 2019) or apple juice (Phaiphan et al., 2019) during storage.
Effect of addition of apple peel extract on TSS values in stored quince juice
The TSS changes of all samples during 15 d of storage at 4°C are shown in Figure 4. The significant changes in the TSS of quince juices were obtained with samples of juice treated with (0.3% and 3%) of alcoholic extracts and 3% of aqueous extracts. They were insignificant after treatment with 0.3% aqueous extracts compared with untreated juices at 0 d of storage. The addition of EAPE with 3% treated juices had TSS values significantly larger than that of EAPE with 0.3% treated samples during all storage periods. Treatment of quince juice with a higher concentration of AAPE was not significant differences compared to lower concentration of AAPE treated juice samples throughout the days of storage. The above results are consistent with those reported by Altunkaya et al. (2013), who studied the effect of pomegranate peel extract on the total soluble solids of apple juice. Another study also reported an increase of TSS when shallot extract was supplemented in apple juice at a concentration ranged between 0.5% and 2% (Phaiphan et al, 2019).
Effect of apple peel extract addition on pH values of quince juice during storage at 4°C
Table 1 demonstrates the pH changes of the quince juice treated with two extracts of apple peel (differing in their extraction method and concentration).
TABLE 1 Effect of addition of alcoholic apple peel extracts to quince juice on pH values.
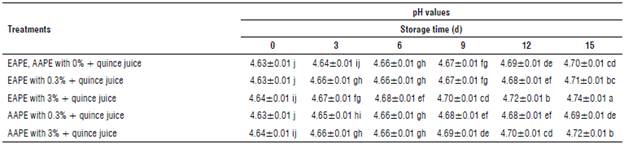
The values In the above table represent means ± standard error and the same letters In each column and rows Indicate no significant differences according to the Duncan test (P>0.05).
The initial pH values of the untreated sample at 0 and 15 d were 4.63 and 4.70, respectively. Insignificant increment in pH values (P>0.05) were obtained immediately after peel extract treatment. From the same table, the EAPE 3% treated juices had a pH value of 4.74 that was significantly larger than EAPE 0.3% treated ones (4.71) after 15 d of storage at 4°C. Samples of quince juice supplemented with different concentrations of aqueous extracts of apple peel were significantly different in the pH at 4.69 and 4.72 at the last time of storage. EAPE and AAPE with concentration 3% had significantly higher pH values than that E APE and AAPE with concentrations of 0.3% and untreated quince juices at a final day of cold storage (Tab. 1). These findings agreed with studies of aonla (or Indian Gooseberry Emblica officinalis Gaertn. Syn. Phyllanthus emblica L.) juice and guava slices in which the pH was increased significantly after plant extracts treatment (Mundhe et al, 2016; Shomodder et al, 2021).
TABLE 2 Effect of addition of alcoholic apple peel extracts to quince juice on titratable acidity values (%).
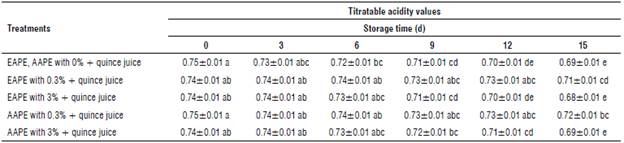
The values in the above table represent mean ± standard error and the same letters in each column and rows indicate no significant differences according to the Duncan test (P>0.05).
Effect of apple peel extract supplementation on titratable acidity content in quince juice during storage
Table 2 illustrates the titratable acidity of the quince juice treated with EAPE, AAPE and untreated juices. Treatment of quince juice with EAPE and AAPE did not exhibit significant differences compared to untreated juice sample at 0 d of storage. The same previous treatment showed significant differences after 15 d of storage ofjuice samples at 4°C. A significant effect of the incorporation of natural extract from apple peel (alcoholic extraction method) into quince juice was shown after 12th d and continued to the end of storage. In the case of water extracts, apple added to quince juice samples during storage under used conditions, the changes in the acidity were not significant until the 15th d of storage. Depending on the concentration of added extracts, quince juices had lower acidity values. This result was similar to that obtained by Mundhe et al. (2016) who observed a reduction of titratable acidity of aonla juice with increasing concentrations of custard apple leaf extract. The EAPE added juice samples had minimum titratable acidity value (0.68), when compared to other treatments.
Similar results were reported by Shomodder et al. (2021) who studied the different characteristics including acidity of guava slices treated with different types of extracts during cold storage for 6 d.
Conclusions
PPO-catalyzed browning is considered a negative phenomenon in fruit juice processing. As far as we know, an application of apple peel extract as PPO inhibitor is the first experimental trial against quince juice browning. It was found that all extracts had the potentiality against browning-related PPO action. Addition of apple extracts to the quince juice improved many properties of the juice compared with extracts free from juice samples during storage for 15 d at 4°C; the increased percentages were (63, 26), (52, 30), (3.20, 4.31), (8.52, 9.98), and (0.22, 2.321), for PPO inhibition, browning index inhibition, TSS, TPC, and pH at 0 and 15 d of storage at 4°C for (3)% EAPE treated quince juice samples compared with control samples, respectively. The extracts used can be ranked as 3% EAPE > 0.3% EAPE > 3% AAPE > 0.3% AAPE. Finally, this study suggested that ethanolic and aqueous apple peel extracts could be utilized as a natural additive in the quince juice processing industry. The production of brown fruit juices caused by some enzymes may led to the appearance of toxic compounds, poor nutritional content, and lower levels of sensory properties. This is a very important processing challenge. To achieve improvement in this respect, it is necessary to investigate some biochemical properties related to safety and quality of these juices. Considering this matter from a future perspective, addition of plant peel extracts as antibrowning agents to the fruit juices should be given more attention.