Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Medicas UIS
versión impresa ISSN 0121-0319
Medicas UIS vol.25 no.3 Bicaramanga sep./dic. 2012
¿What is the evidence, from behavioural and
imaging data, that we can predict how well
people recover after ischemic stroke?
Álvaro Rojas Villabona*
*MD. MSc Clinical Neuroscience. Institute of Neurology. University College London. London. United Kingdom.
Correspondence: Insitute of Neurology. University College London. Queen Square. London. WC1N 3BG, United Kingdom. e-mail: ca.villabona.11@ucl.ac.uk.
Article received the 17th of September of 2012 and accepted for publication the 17th of december of 2012.
ABSTRACT
Background: the incidence of stroke in developing countries is increasing and it is the leading cause of longstanding disability in developed countries. Early prediction of future functional abilities is important for stroke management. It is intended to review whether the initial severity of the deficit and the imaging findings could predict long term recovering after ischemic stroke. Methods: the PubMed database was searched to identify studies evaluating how the initial neurological deficit and the imaging findings could predict long term recovery after ischemic stroke. 35 articles were selected to develop a non systematic review and the Oxford Centre for Evidence-based Medicine Levels of Evidence model was used to grade the quality of the found evidence. Results: age and initial deficit evaluated with the National Institutes of Health Stroke Scale were the best predictors of long term recovery after ischemic stroke. The severity of the deficit in specific categories such as upper limb functions, walking and activities of the daily life had a lower level of evidence on prediction of post-stroke disability. Not a definite prognostic value had been convincingly demonstrated for size of infarction. Location of the lesion, particularly the compromise of the cortico-spinal tract evaluated with diffusion tensor imaging appeared to be a good predictor of recovery, and the pattern of brain activation after stroke evaluated with functional magnetic resonance imaging or positron emission tomography scan had a moderate level of evidence as predictor of recovery after stroke. Conclusion: the severity of the initial deficit can be used to predict how well subjects will recover from an ischemic stroke and novel imaging techniques are very promising tools to predict long time recovery after ischemic stroke. (MÉD.UIS. 2012;25(3):229-38)
Keywords: Stroke. Forecasting. Recovery of Function. Diagnostic Imaging.
¿Cuál es la evidencia, de comportamiento y datos de imágenes, que podemos predecir címo se recupera la gente
después de un accidente cerebrovascular isquémico?
RESUMEN
Introducciín: la incidencia de enfermedad cerebro vascular en países desarrollados está en aumento y es la primera causa de discapacidad permanente en países desarrollados. La predicciín temprana de futura funcionalidad es importante para el tratamiento de la enfermedad cerebro vascular. Se pretende revisar si la severidad del déficit inicial y los hallazgos radiolígicos podrían predecir la recuperaciín funcional a largo plazo tras un accidente cerebro vascular isquémico. Metodología de búsqueda: se desarrollí una búsqueda bibliográfica en la base de datos PubMed, para identificar estudios que evalúen címo el déficit neurolígico inicial y los hallazgos radiolígicos pueden predecir la recuperaciín a largo plazo en accidente cerebro vascular isquémico. Se seleccionaron 35 artículos para desarrollar una revisiín no sistemática de la literatura y se usí como modelo de niveles de evidencia del centro de medicina basada en la evidencia de Oxford, para evaluar la calidad de la literatura encontrada. Resultados: la edad y el déficit inicial evaluado con la escala de enfermedad cerebro vascular de los Institutos Nacionales de Salud, fueron los mejores predictores de recuperaciín a largo plazo tras un accidente cerebro vascular isquémico. La severidad del déficit en categorías específicas, como por ejemplo, funciín del miembro superior, marcha y actividades de la vida diaria, tuvieron un nivel menor de evidencia en predicciín de discapacidad posaccidente cerebro vascular. Un valor pronístico definitivo para el tamaño del infarto no ha sido convincentemente demostrado. La localizaciín de la lesiín, particularmente el compromiso del tracto cortico espinal evaluado con imágenes de difusiín por tensiín, parece ser un buen predictor de recuperaciín. El patrín de activaciín cerebral tras un accidente cerebro vascular evaluado con resonancia magnética funcional y tomografía por emisiín de positrones tuvo un moderado nivel de evidencia como predictor de recuperaciín tras un accidente cerebro vascular. Conclusiones: la severidad del déficit inicial puede ser usado para predecir recuperaciín neurolígica tras un accidente cerebro vascular isquémico y nuevas técnicas radiolígicas son muy prometedoras en la predicciín de recuperaciín a largo plazo de la enfermedad cerebro vascular isquémica. (MÉD.UIS.2012;25(3):229-38)
Palabras Clave: Accidente cerebrovascular. Predicciín. Recuperaciín de la Funciín. Diagnístico por Imagen.
BACKGROUND
Stroke is the most common cause of acquired adult disability in developed countries1. Estimation of potential recovery is essential in clinical settings, in order to establish realistic rehabilitation goals and planning the type and duration of health care and community support2. Several factors have been shown useful predicting stroke outcome; however inter-individual factors make accurate prediction very difficult and there is not any model or any technique is largely accepted or routinely used to predict long term recovery on ischemic stroke patients3. Several neurological domains can be affected by stroke; motor function and mobility, language, and Activities of Daily Life (ADL) are the most common used outcomes; and the prognosis of recovery on every of those aspects is influenced by a different set of factors. Additionally, factors predicting recovery are influenced by the time of assessment and kind of stroke; which make studies on this field very heterogeneous and their comparability very poor4. Evaluation of quality of the available evidence is extremely important given the huge amounts of published literature and the continuous and fast development on imaging techniques. It is intended to evaluate how good is the initial severity of the deficit and the imaging findings at predicting long time recovering after ischemic stroke.
METHODS
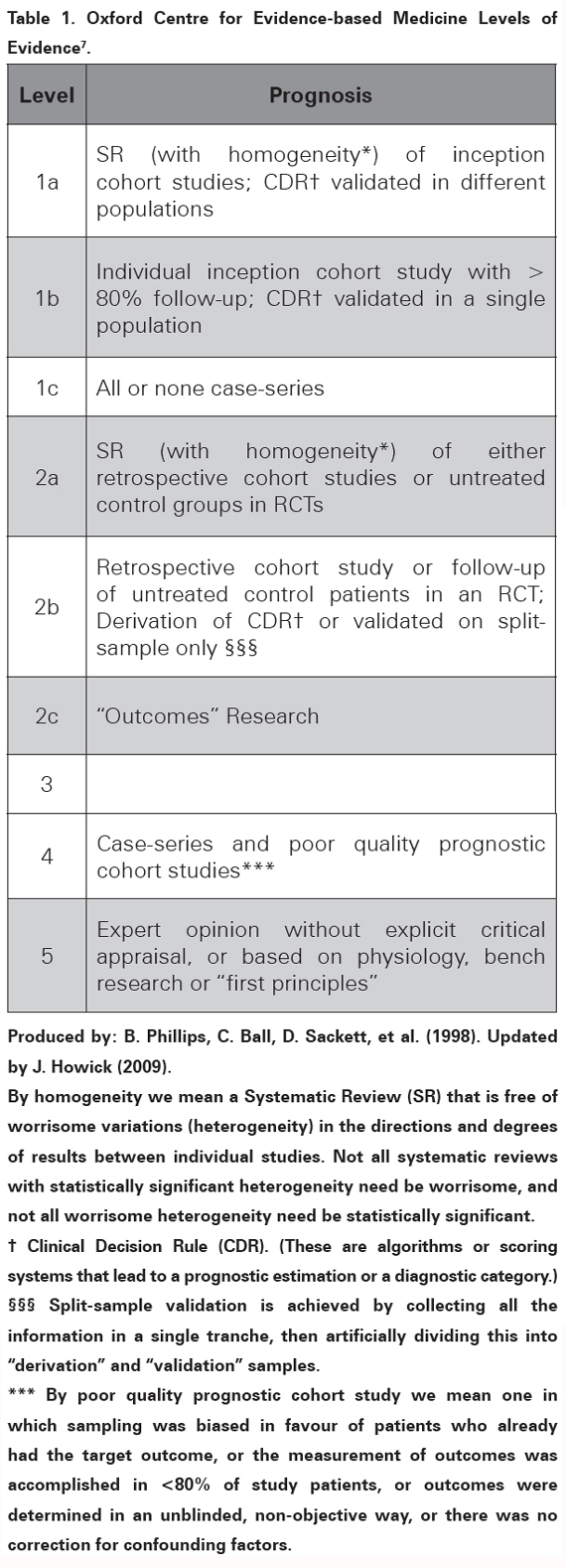
Search: a literature search was performed to identify studies evaluating how the initial neurological deficit and the imaging findings could predict long term recovery after ischemic stroke. The PubMed database (US National Library of Medicine) was searched using the keywords: "stroke" or "cerebral infarction" and "imaging" or "MRI" or "predictors" and "prognosis" or "recovery of function" or "rehabilitation" or "outcome prediction". The search was limited to studies on adult humans and papers published after 2005 in English or Spanish language. The initial PubMed search retrieved 1028 articles. The titles and abstracts of those articles were reviewed and an initial selection was done excluding papers evaluating impact of any kind of treatment on patient outcome as well as studies with poor follow up or that included patients with haemorrhagic strokes. Afterwards, 35 articles were considered relevant and their quality assessed to carry out a review on prediction of recovery after ischemic stroke. Evaluation of evidence: countless groups around the world work on prognosis and prediction of recovery after stroke, hence the massive amounts of published evidence. The optimal evidence for prognostic factors comes from Systematic Reviews (SR) of homogeneous inception cohort studies or scoring systems validated in different populations5. However, the majority of the found papers are SR with qualitative results; not validated scoring systems, and cohort studies with design and quality issues that need to be well evaluated and interpreted in order to translate their findings to the clinical grounds6. The Oxford Centre for Evidence-based Medicine Levels of Evidence model, updated in 20097 (see Table 1); has a specific section for studies on prognosis which has been shown applicable to studies on prediction of recovery in stroke8. It will be used across this manuscript to grade the quality of the available evidence; based on personal appraisal of five main aspects: population features, evaluated prognostic factors and outcomes, use of comparison groups and study design.
RESULTS
Prediction of recovery scales Multiple scales and scoring systems have been proposed to accurately predict functional recovery after stroke, but not any Clinical Decision Rules (CDR) has been rigorously validated in different populations; and reached enough level of evidence to be routinely used9,10. However, studies doing scales and predicting scores have been essential to demonstrate that the initial severity of the deficit measured with the score of the National Institutes of Health Stroke Scale (NIHSS) can predict long term recovery after stroke. The NIHSS is a simple systematic assessment tool that provides quantitative measure of stroke-related neurologic deficit. It is based on clinical examination of consciousness, motor and sensory function, visualfields, coordination, language and attention; takes less than ten minutes to complete and the scores range from 0 to 42, with 0 as normal11.
The fundamental prognostic importance of NIHSS was stated in a study in Germany; they proposed and validated an early prognostic mathematical index based on two variables, age and NIHSS, which could predict correctly 83.2 % of the patients who had complete functional recovery [Barthel Index (BI) > 95] and 91.5 % of the surviving patients 100 days after the stroke11. BI is an ordinal quantitative scale used to measure performance in ADL. External factors from the environment affect the score of each item. A higher score is associated with a greater likelihood of being able to live at home with a certain degree of independence following discharge. It is used as an outcome to evaluate long term disability in different conditions12. That was a well-designed inception cohort study validated in one population with a follow up rate of 83.3 % (level of evidence 1b); nonetheless, it did not evaluate the predictive value of NIHSS independently11. More recently, two attempts to produce CDR scales have reconfirmed the value of NIHSS to predict long term recovery of stroke. Vora et al. developed a scale to predict outcome after cortical middle cerebral artery infarction, and validated it in an independent population11. Five independent predictors of outcome were found: age, NIHSS (OR, 1.17; 95% CI, 1.06 to 1.30; P: 0.003), infarct volume, admission white blood cell count and presence of hyperglycaemia. It was a retrospective, single centre, cohort study (129 patients) that defined the outcome as a modified Ranking Score (mRS) of > 2 at 30 days and used multivariable analysis to assess independency of the predictor factors, level of evidence 2b13. The mRS is a scale useful for measuring the degree of disability or dependence in the ADL of patients who have suffered of a stroke. It is now the most widely used clinical outcome measure on stroke research. The scale runs from 0 to 6, with zero as perfect health and 6 as death14.
Similarly, Muscari et al developed a simple scoring system for prediction of recovery at nine months after the stroke. Five risk factors were found significant and they compose the Bologna Outcome Algorithm for Stroke. The included prognosis factors where again NIHSS ≥ and age ≥78, accompanied by the need of urinary catheter, oxygen administration, and persistence of upper limb paralysis at discharge. This was a retrospective cohort study (221 patients) with ischemic stroke not undergoing thrombolysis, 10.8 % of missing data; and validated in a group of 100 patients of the same institution; level of evidence 2b15. As it can be seen one of the studies evaluated the patients at one month, the other one at 100 days and the last one at 9 months, it make them not only no comparable but more important, it rises the importance of time on evaluation of prognostic factors and outcomes in studies of stroke recovery 12,16.
Despite the poor quality of the studies, cumulative evidence support the predictive value of NIHSS17,18; furthermore, it has been shown suitable to evaluate and predict recovery of different aspects such as motor function, ADL and language9,19. NIHSS can be easily done in the bedside and seems not to be highly influenced by the time of evaluation in the first nine days post stroke which makes of it a high quality predictive tool20.
Two important points are extracted from studies on scoring systems; first, age is the strongest predictor of recovery in all kind of stroke and is generally accepted in the clinical and research field6,9,21. Secondly, the evidence for stroke size is controversial and not enough to support CDR; we will come back to it on the section of imaging studies.
PROGNOSIS FACTORS IN DIFFERENT DOMAINS
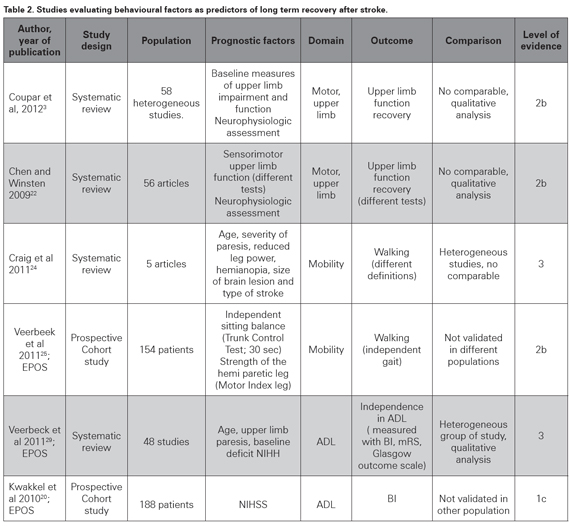
Not all the studies produce scoring models using global evaluations, but some of them evaluate single prognostic factors that could impact recovery in specific domains as motor function, independency or ADL and language function (see Table 2). Two systematic reviews have been done to evaluate prediction of upper motor limb function recovery after stroke. Chen et al systematically reviewed the literature to identify the best predictors of arm-specific motor recovery. They assessed methodological quality of 56 studies published between 1979 and 2008 using a score system based on internal, statistical and external validity; and found that only 36% of them were of high methodological quality (score ≥10 of 15). Analysis with the best evidence criteria showed initial neurophysiologic factors and initial motor capability as the best predictors of upper limb motor recovery22. The most important issue raised in the statistical analysis was the heterogeneity of the systems used to evaluate the initial deficit and the scales measuring the outcomes in the studies; which made a quantitative analysis of prognostic factors impossible. More recently Coupr and others reviewed and summarized 58 poor quality studies to conclude that baseline measures of upper limb impairment and function were significant predictors of upper limb recovery3; OR 14.84 (95% CI 9.08–24.25) and 38.62 (95% CI 8.40–177.53), respectively. Again, the interpretation of the results is complicated by methodological factors such as variations in study populations, the selected predictors, the used outcome scales, and timing of baseline and outcome assessments23. Those SR reached similar results and that evidence is very important and conclusive; however their level of evidence is still not optimal (2b, see Table 2), and more homogeneous cohort studies are needed to definitely demonstrate that initial measures of upper limb function and impairment, and neurophysiological measures can predict upper limb recovery3.
Another landmark for motor recovery is mobility; particularly walking, which highly impacts independence and functionality and was the scope of a SR made by Craig et al. A group of five heterogeneous, non-comparable studies evaluating patients within one week post stroke were included; they did not meet the majority of the criteria for good prognostic research and only two of them developed a prognostic model based on multivariate analysis. Walking was evaluated for all the studies, but their outcome measure systems were different. They found that age, severity of paresis, reduced leg power, presence of hemianopia, size of brain lesion and type of stroke were predictive or associated with walking within 30 days post-stroke24. That study can be described as a SR because it used a planned search strategy; however, the analysis was qualitatively done and not any integrating parameter was obtained. The Oxford model of evidence grading does not have a specific level for heterogeneous SR, that is why the formerly described SR were arbitrarily assigned a level of evidence 2b; however, the latter paper does not even reach that level of evidence (level of evidence 3, see Table 2). By the time that review was being done, the Early Prediction of functional Outcome after Stroke (EPOS) group was working on a prospective, multicenter cohort study to evaluate if independent gait at six months can be accurately predicted based on simple clinical tests within the first 72 hours. A group of 154 first-ever ischemic stroke patients unable to walk independently were assessed and a multivariable logistic model was used to identify prognostic factors for regaining independent gait. Patients with an independent sitting balance (Trunk Control Testsitting; 30 seconds) and strength of the hemiparetic leg (Motricity Index leg) on day two poststroke had a 98% probability of achieving independent gait at 6 months compared with a probability of 27% in patients who were unable to sit independently for 30 seconds and were hardly or not able to contract the muscles of the paretic lower limb25. It was a well-designed study with 85 % follow up; and their conclusion are well founded, however the absence of external validation detract its evidence strength (level of evidence 2b). It is important to point out that this recently designed study is neither comparable to the ones included in the aforementioned SR; due to different scales used to measure function and outcome. The results are concordant with other reports26,27, and in general support the idea that severity of deficit, in this case measured as strength of the hemiplegic leg and sitting balance, can accurately predict recovery of mobility and specifically, walking9,28.
ADL scales have been widely used to evaluate recovery since they describe the actual impact that a deficit can have on functionality. A SR was done by the EPOS group to identify factors in the first two weeks post-stroke that are predictive for outcome of ADL three months after stroke. A synthesis was performed of forty-eight studies; the insufficient methodological quality of most prognostic studies made a quantitative analysis infeasible (median risk of bias score: 17 out of 27; range 6–22). Nevertheless, six high-quality studies coincided that baseline neurological status (measured with the NIHSS or Canadian Neurological Scale), upper limb paresis, and age are predictors for outcome of ADL beyond three months post-stroke (level of evidence 3)29. Those data are consistent with the shown evidence and in general support the value of the initial severity measures, particularly the NIHSS to predict long term post stroke outcome in terms of BI20.
IMAGING STUDIES
Imaging is essential for assessment of stroke patients, it differentiates ischemic from haemorrhagic stroke, orientates on the cause of the disease and gives an estimate of the size of the lesion; the latter, has been largely studied but optimal quality evidence is lacking to support a long-term recovery predictive value for infarct size3,30. A review of infarct volume measured with a Magnetic Resonance Imaging (MRI) for prediction of recovery reported 13 studies using different MRI techniques such as T2 Weighted imaging (T2WI), Diffusion Weighted Imaging (DWI) and Perfusion Weighted Imaging (PWI)31. Most of the studies satisfied the methodological criteria for adequate prognostic research, but none of them took lesion location into account and their heterogeneity made the analysis not conclusive. However, correlation coefficients between MRI lesion volume and outcomes seem to be better for outcomes defined through clinical evaluation (NIHSS; median 0.67; range: 0.57–0.91) than for those at the functionality level31.
Many studies support a positive predictive value for infarct size13,32, and recently the prospective Acute Stroke Accurate Prediction study showed that repetitive measurement of lesion size could increase the accuracy of recovery prediction at three months (level of evidence 2c)32. However, an equally large and repetitive set of evidence report the lack of predictive value for infarct size, particularly highlighted, has been the absence of any added value compared to age and initial neurological deficits3,4,33. The main issue raised on lesion size as predictor of recovery is which technique should be used to measure it, different neuroimaging methods are known to produce different lesion volumes and their predictive values differ34.
Computerized tomography is the most commonly used imaging technique on stroke units but is not a good method to measure infarct size. Conventional T2W MRI could reliably detect lesions within one and eight hours after the ischemia onset and have been proposed as a more reliable method for long-term prediction35. DWI detects water diffusion restriction in the tissue even in the first hour after the stroke36. The most severe diffusion restrictions occur in the centre of the perfusion deficit and that could be used to discriminate between viable and definitely necrotic tissue. A SR was done to evaluate if DWI represents irreversibly infarcted tissue (ischemic core) in acute stroke. Evaluation of 61 studies was done using the criteria set of the Oxford Centre for Evidence-Based Medicine and only a small number of them had level of evidence 1 or 2. The analysis showed a high variability in the studies and a surprisingly high mean rate of DWI lesion reversal (24%) which was interpreted as inconsistent with the tissue outcome (level of recommendation D, level 5 evidence or troublingly inconsistent)8. PWI measures vascular supply to the tissues, which is an early phenomenon (within minutes) in the physiopathologic cascade of stroke probably associated with the outcome31. Lesion size estimates using PWI and DWI differ markedly and that difference is being studied as an indirect measure of the penumbra area (mismatch) and a possible outcome predictor37. Recanalization, both therapeutic and spontaneous can influence the size of the infarct and not necessarily the recovery of the patient; that is why methods evaluating blood flow such as magnetic resonance angiography have failed to show long time recovery38.
The evaluation of lesion size as a predictor of recovery is greatly affected by the heterogeneity of the studies37 and the available evidence is not significant enough to support a predictive value. Instead, the evidence suggest that big lesions in some specific areas can predict long term disability, which indicate the value of infarct location and the damage to specific tracts as predictors of recovery30.
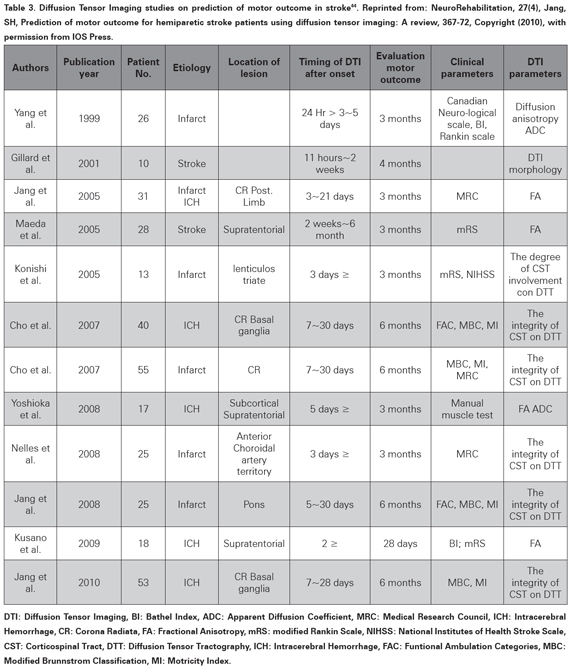
Descending motor tracts, especially the Cortico- Spinal Tract (CST) integrity is essential for gross motor trunk and limb function39,40. Diffusion Tensor Imaging (DTI)41 and Diffusion Tensor Tractography (DTT)42 have been used to evaluate the integrity of the CST and its compromise has being proposed as a predictive factor for poor outcome43. A recent review (not systematic) evaluated 12 studies and suggested a predictive value for long term recovery for CST integrity assessed with DTI (see Table 3)44, which is similar to other papers not included in that review45,46. However the quality of the individual papers and the review is very poor and cannot support a prognostic value47,48(level of evidence 5).
A recent well designed study evaluated 60 patients with DTT within 12 hours of middle cerebral artery stroke and found that CST damage at the level of the posterior limb of the internal capsule is a significant predictor of unfavourable motor outcome assessed with the motor sub-index scores of the NIHSS at the day 90 (sensitivity 73.7%; specificity: 100%)4.
Furthermore, DTT predicted motor outcome at day 90 better than the clinical scores4, which needs to be validated in other populations (level of evidence 2b). Predictability of DTT for motor outcome can differ according to the time and seems to be better when measured after two weeks49. The aforementioned studies show a weak positive predictive value for DTI CST integrity (level of evidence 2)50; but no conclusive SR has being done to support a value superior to age and NIHSS need to be proved as well4. This approach of infarct location to predict recovery is extending to other functions, and the use of imaging to predict recovery is extending to multiple and specific areas of disability, such as language and dysphagia51.
Functional imaging of the brain involves a group of techniques that detect metabolic changes and then activation in cerebral tissues. It has been proposed that activation pattern of the brain after ischemic lesion could predict long term recovery52. A SR evaluated studies using functional MRI (fMRI) and positron emission tomography to see how changes in brain activity after stroke could predict recovery within six months post-stroke30. Twenty-two studies, which satisfied the basic methodological criteria, investigated the association between task-related brain activation patterns and functional recovery of the upper limb. They found profound cerebral reorganization occurring after stroke, including overactivation of primary and association motor areas, posterior shift in activity in the primary motor cortex and bilateral recruitment of non-motor areas and those changes seem to diminish linearly with the neurological recovery30. Quantitative analysis was impossible due to lack of consensus on outcome measures; however, a trend of activation pattern was correlated to long term recovery (level of evidence 2b). In patients with favourable recovery, these overactivations are transient, while in poorly recovering patients there seems to be a persistent recruitment of contralateral motor and associative areas35.
The studies on prognostic factor for post-stroke recovery are very heterogeneous concerning the kind of stroke included and the treatment given to the patients. Several studies have been carried out, on prediction of recovery in patients who received recombinant tissue plasminogen activator53. Imaging has not convincingly shown to have significant strong evidence to support a predictive value on longterm recovery, however, combination of imaging techniques and behavioural evaluations seems to be more predictive than behavioural measures alone10,52.
CONCLUSION
The prediction of long term recovery after ischemic stroke is important for management of stroke patients and heterogeneity of the studies is the main issue for generalization and applicability of predictor factors. Age and initial deficit evaluated with the NIHSS scale are the best predictors of long term recovery after ischemic stroke based on cumulative evidence. However, the optimal (1a) level of prognostic evidence has not been reached. The severity of deficit in specific categories such as upper limb function, walking and ADL have a lower level of evidence on prediction of post-stroke disability (level of evidence less than 2b), explained by heterogeneity on populations, prognostic factors, time of evaluation and follow up, outcome scales and statistical analysis used. Nevertheless, the evidence described is enough to affirm that the severity of the initial deficit can be used to predict how well people will recover from an ischemic stroke. Sample size as a prognostic factor in stroke has been largely studied, but the evidence is very conflicting and not a definite prognostic value has been convincingly demonstrated for size of infarction. The new imaging techniques are very promising for prediction of recovery after stroke and a massive amount of research is being done on them. DWI and PWI seem to be capable of evaluating outcome of tissue in the penumbra area, but it has not been shown to correlate long term recovery. Location of the lesion, particularly the compromise of the CST evaluated with DTI appear to be a good predictor of recovery, and the pattern of brain activation after stroke evaluated with fMRI or positron emission tomography scan has a moderate level of evidence as predictors of recovery after ischemic stroke. Still, a predictive value superior to age and initial NIHSS need to be proven. Prognosis is a critical component of the management decision making process. Optimal levels of evidence on prediction factors should support aggressive therapies for patients with good prognosis as well as to question the role of aggressive management for patients that will not do well regardless of the offered management. Development of homogeneous cohort studies with appropriate follow up and comparable evaluation scales should provide high levels of evidence to develop more personalized management guidelines.
REFERENCIAS BIBLIOGRÁFICAS
1. Adamson J, Beswick A, Ebrahim S. Is stroke the most common cause of disability? J Stroke Cerebrovasc Dis. 2004;13(4):171-7. [ Links ]
2. Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9(12):1228-32. [ Links ]
3. Coupar F, Pollock A, Rowe P, Weir C, Langhorne P. Predictors of upper limb recovery after stroke: a systematic review and metaanalysis. Clin Rehabil. 2012;26(4):291-313. [ Links ]
4. Puig J, Pedraza S, Blasco G, Daunis-I-Estadella J, Prados F,Remollo S, et al. Acute damage to the posterior limb of the internalcapsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol. 2011;32(5):857-63. [ Links ]
5. Phillips B, Ball C, Badenoch D, Straus S, Haynes B, Dawes M. Oxford centre for evidence-based medicine levels of evidence (May 2001). BJU International. 2011;107(2):348. [ Links ]
6. Counsell C, Dennis M. Systematic review of prognostic models in patients with acute stroke. Cerebrovasc Dis. 2001;12(3):159-70. [ Links ]
7. Phillips B BC, Sackett D. Oxford Centre for Evidence-based Medicine Levels of Evidence. 2001. Updated by J. Howick 2009. Disponible en: hhttp://www.cebm.net/?o=1025. [ Links ]
8. Kranz PG, Eastwood JD. Does diffusion-weighted imaging represent the ischemic core? An evidence-based systematic review. AJNR Am J Neuroradiol. 2009;30(6):1206-12. [ Links ]
9. Hallevi H, Albright KC, Martin-Schild SB, Barreto AD, Morales MM, Bornstein N, et al. Recovery after ischemic stroke: criteria for good outcome by level of disability at day 7. Cerebrovasc Dis. 2009;28(4):341-8. [ Links ]
10. Alexander LD, Pettersen JA, Hopyan JJ, Sahlas DJ, Black SE. Long-term prediction of functional outcome after stroke using the Alberta stroke program early computed tomography score in the subacute stage. J Stroke Cerebrovasc Dis. 2012;21(8):737-44. [ Links ]
11. Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC. Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke. 2004;35(1):158-62. [ Links ]
12. Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61-3. [ Links ]
13. Vora NA, Shook SJ, Schumacher HC, Tievsky AL, Albers GW, Wechsler LR, et al. A 5-item scale to predict stroke outcome after cortical middle cerebral artery territory infarction: validation from results of the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke. 2011;42(3):645-9. [ Links ]
14. Wilson JT, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33(9):2243-6. [ Links ]
15. Muscari A, Puddu GM, Santoro N, Zoli M. A simple scoring system for outcome prediction of ischemic stroke. Acta Neurol Scand. 2011;124(5):334-42. [ Links ]
16. Knoflach M, Matosevic B, Rücker M, Furtner M, Mair A, Wille G, et al. Functional recovery after ischemic stroke--a matter of age: data from the Austrian Stroke Unit Registry. Neurology. 2012;78(4):279-85. [ Links ]
17. Jeng JS, Huang SJ, Tang SC, Yip PK. Predictors of survival and functional outcome in acute stroke patients admitted to the stroke intensive care unit. J Neurol Sci. 2008;270(1-2):60-6. [ Links ]
18. Hemmen TM, Ernstrom K, Raman R. Two-hour improvement of patients in the National Institute of Neurological Disorders and Stroke trials and prediction of final outcome. Stroke. 2011;42(11):3163-7. [ Links ]
19. Bland MD, Beebe JA, Hardwick DD, Lang CE. Restricted active range of motion at the elbow, forearm, wrist, or fingers decreases hand function. J Hand Ther. 2008;21(3):268-74. [ Links ]
20. Kwakkel G, Veerbeek JM, van Wegen EE, Nijland R, Harmelingvan der Wel BC, Dippel DW. Predictive value of the NIHSS for ADL outcome after ischemic hemispheric stroke: does timing of early assessment matter?. J Neurol Sci. 2010;294(1-2):57-61. [ Links ]
21. Muto Y, Uchimura M, Waki S, Hayashi T, Samejima K, Okamoto K. Clinicopathologic study of adenosquamous carcinoma of the gallbladder and bile duct. Jpn J Cancer Clin. 1982;28:440-4. [ Links ]
22. Chen SY, Winstein CJ. A systematic review of voluntary arm recovery in hemiparetic stroke: critical predictors for meaningful outcomes using the international classification of functioning, disability, and health. J Neurol Phys Ther. 2009;33(1):2-13. [ Links ]
23. Nijland RH, van Wegen EE, Harmeling-van der Wel BC, Kwakkel G. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke. 2010;41(4):745-50. [ Links ]
24. Craig LE, Wu O, Bernhardt J, Langhorne P. Predictors of poststroke mobility: systematic review. International journal of stroke : official journal of the International Stroke Society. 2011;6(4):321-7. [ Links ]
25. Veerbeek JM, Van Wegen EE, Harmeling-Van der Wel BC, Kwakkel G. Is accurate prediction of gait in nonambulatory stroke patients possible within 72 hours poststroke? The EPOS study. Neurorehabil Neural Repair. 2011;25(3):268-74. [ Links ]
26. Kollen B, Kwakkel G, Lindeman E. Longitudinal robustness of variables predicting independent gait following severe middle cerebral artery stroke: a prospective cohort study. Clin Rehabil. 2006;20(3):262-8. [ Links ]
27. Verheyden G, Nieuwboer A, De Wit L, Feys H, Schuback B, Baert I, et al. Trunk performance after stroke: an eye catching predictor of functional outcome. J Neurol Neurosurg Psychiatry. 2007;78(7):694-8. [ Links ]
28. Patel AT, Duncan PW, Lai SM, Studenski S. The relation between impairments and functional outcomes poststroke. Arch Phys Med Rehabil. 2000;81(10):1357-63. [ Links ]
29. Veerbeek JM, Kwakkel G, van Wegen EE, Ket JC, Heymans MW. Early prediction of outcome of activities of daily living after stroke: a systematic review. Stroke. 2011;42(5):1482-8. [ Links ]
30. Kissela B, Lindsell CJ, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, et al. Clinical prediction of functional outcome after ischemic stroke: the surprising importance of periventricular white matter disease and race. Stroke. 2009;40(2):530-6. [ Links ]
31. Butcher K, Parsons M, Baird T, Barber A, Donnan G, Desmond P, et al. Perfusion thresholds in acute stroke thrombolysis. Stroke. 2003;34(9):2159-64. [ Links ]
32. Barrett KM, Ding YH, Wagner DP, Kallmes DF, Johnston KC. Change in diffusion-weighted imaging infarct volume predicts neurologic outcome at 90 days: results of the Acute Stroke Accurate Prediction (ASAP) trial serial imaging substudy. Stroke. 2009;40(7):2422-7. [ Links ]
33. Wardlaw JM, Keir SL, Bastin ME, Armitage PA, Rana AK. Is diffusion imaging appearance an independent predictor of outcome after ischemic stroke?. Neurology. 2002;59(9):1381-7. [ Links ]
34. Tong DC, Yenari MA, Albers GW, O'Brien M, Marks MP, Moseley ME. Correlation of perfusion- and diffusion-weighted MRI with NIHSS score in acute (<6.5 hour) ischemic stroke. Neurology. 1998;50(4):864-70. [ Links ]
35. Buma FE, Lindeman E, Ramsey NF, Kwakkel G. Functional neuroimaging studies of early upper limb recovery after stroke: a systematic review of the literature. Neurorehabil Neural Repair. 2010;24(7):589-608. [ Links ]
36. Yoo AJ, Barak ER, Copen WA, Kamalian S, Gharai LR, Pervez MA, et al. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with National Institutes of Health Stroke Scale Score improves the prediction of acute stroke outcome. Stroke. 2010;41(8):1728-35. [ Links ]
37. Farr TD, Wegener S. Use of magnetic resonance imaging to predict outcome after stroke: a review of experimental and clinical evidence. J Cereb Blood Flow Metab. 2010;30(4):703-17. [ Links ]
38. Kumar A, Anel R, Bunnell E, Zanotti S, Habet K, Haery C, et al. Preload-independent mechanisms contribute to increased stroke volume following large volume saline infusion in normal volunteers: a prospective interventional study. Crit Care. 2004;8(3):128-36. [ Links ]
39. Rapisarda G, Bastings E, de Noordhout AM, Pennisi G, Delwaide PJ. Can motor recovery in stroke patients be predicted by early transcranial magnetic stimulation? Stroke. 1996;27(12):2191-6. [ Links ]
40. Cho SH, Kim DG, Kim DS, Kim YH, Lee CH, Jang Sh. Motor outcome according to the integrity of the corticospinal tract determined by diffusion tensor tractography in the early stage of corona radiata infarct. Neurosci Lett. 2007;426(2):123-7. [ Links ]
41. Kusano Y, Seguchi T, Horiuchi T, Kakizawa Y, Kobayashi T, Tanaka Y, et al. Prediction of functional outcome in acute cerebral hemorrhage using diffusion tensor imaging at 3T: a prospective study. AJNR Am J Neuroradiol. 2009;30(8):1561-5. [ Links ]
42. Nelles M, Gieseke J, Flacke S, Lachenmayer L, Schild HH, Urbach H. Diffusion tensor pyramidal tractography in patients with anterior choroidal artery infarcts. AJNR Am J Neuroradiol. 2008;29(3):488-93. [ Links ]
43. Schiemanck SK, Kwakkel G, Post MW, Kappelle LJ, Prevo AJ. Impact of internal capsule lesions on outcome of motor hand function at one year post-stroke. J Rehabil Med. 2008;40(2):96-101. [ Links ]
44. Jang SH. Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: A review. NeuroRehabilitation. 2010;27(4):367-72. [ Links ]
45. Pineiro R, Pendlebury ST, Smith S, Flitney D, Blamire AM, et al. Relating MRI changes to motor deficit after ischemic stroke by segmentation of functional motor pathways. Stroke. 2000;31(3):672-9. [ Links ]
46. Lee JS, Han MK, Kim SH, Kwon OK, Kim JH. Fiber tracking by diffusion tensor imaging in corticospinal tract stroke: Topographical correlation with clinical symptoms. Neuroimage. 2005;26(3):771-6. [ Links ]
47. Maeda T, Ishizaki K, Yura S. [Can diffusion tensor imaging predict the functional outcome of supra-tentorial stroke?]. No To Shinkei. 2005;57(1):27-32. [ Links ]
48. Yang Q, Tress BM, Barber PA, Desmond PM, Darby DG, Gerraty RP, et al. Serial study of apparent diffusion coefficient and anisotropy in patients with acute stroke. Stroke. 1999;30(11):2382-90. [ Links ]
49. Kwon YH, Jeoung YJ, Lee J, Son SM, Kim S, Kim C, et al. Predictability of motor outcome according to the time of diffusion tensor imaging in patients with cerebral infarct. Neuroradiology. 2012;54(7):691-7. [ Links ]
50. Puig J, Pedraza S, Blasco G, Daunis IEJ, Prats A, Prados F, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol. 2010;31(7):1324-30. [ Links ]
51. Flowers HL, Skoretz SA, Streiner DL, Silver FL, Martino R. MRI-based neuroanatomical predictors of dysphagia after acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis. 2011;32(1):1-10. [ Links ]
52. Zarahn E, Alon L, Ryan SL, Lazar RM, Vry MS, Weiller C, et al. Prediction of motor recovery using initial impairment and fMRI 48 h poststroke. Cereb Cortex. 2011;21(12):2712-21. [ Links ]
53. Sillanpää MJ, Pikkuhookana P, Abrahamsson S, Knürr T, Fries A, Lerceteau E, et al. Simultaneous estimation of multiple quantitative trait loci and growth curve parameters through hierarchical Bayesian modeling. Heredity (Edinb). 2012;108(2):134-46. [ Links ]