Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Iatreia
versión impresa ISSN 0121-0793
Iatreia v.22 n.2 Medellín abr./jun. 2009
ARTÍCULO DE REVISIÓN
Management of maturity–onset diabetes of the young (MODY)
Diego Botero1
1 Attending Physician, Division of Pediatric Endocrinology, Miami Children's Hospital.
Contacto: Diego.Botero@mch.com
Resumen
La diabetes de tipo MODY (maturity–onset diabetes of the young) afecta entre 1 y 5% de los pacientes con diabetes en los Estados Unidos y otras naciones industrializadas. Las tres características más importantes de esta entidad son: desarrollo de diabetes antes de la edad de 25 a 30 años en ausencia de autoanticuerpos pancreáticos, transmisión genética autosómica dominante y evidencia de secreción residual de insulina. Existen seis subtipos de MODY de los cuales, el tipo 2 (mutación de la glucoquinasa–GKS) y el tipo 3 (mutación del factor nuclear hepático 1 alfa (HNF–1–a) son los más prevalentes (70% de todos los casos de diabetes de tipo MODY). Las sulfonilureas son la medicación de primera línea tanto en los niños como en los adultos, cuando la terapia dietética no es suficiente para normalizar la glicemia. Aunque los pacientes con subtipos 1, 3, y 4 usualmente responden bien a la terapia oral con sulfonilureas, un porcentaje significativo de pacientes con los subtipos 1 y 3 necesitan terapia con insulina debido a un deterioro progresivo de las células beta del páncreas. El mantenimiento de un estilo de vida activo y un peso normal, son recomendaciones esenciales en todos los pacientes con diabetes de tipo MODY.
Palabras clave: Diabetes, Insulina, MODY, Sulfonilureas
SUMMARY
Maturity–Onset Diabetes of the Young (MODY) affects 1–5% of people with diabetes in the USA and other industrialized countries. The three main features of MODY include: Development of diabetes before the age of 25 to 30 in absence of pancreatic antibodies, autosomal dominant inheritance, and evidence of residual insulin secretion. There are six subtypes of MODY of which, MODY2 (GCK mutation) and MODY3 (HNF1–a mutation) are the most prevalent, accounting for more than 70% of cases. Sulfonylureas (SUs) remain the medication of first choice in children and adults when dietary therapy is insufficient to maintain normoglycemia. Although patients with MODY1, 3, and 4 usually respond very well to oral SUs, due to progressive ß–cell failure, a significant proportion of MODY1 and MODY3 patients may eventually require insulin therapy. Leading an active lifestyle and maintaining a normal weight are essential recommendations for all MODY patients.
Key words: Diabetes, Insulin, MODY, Sulfonylureas
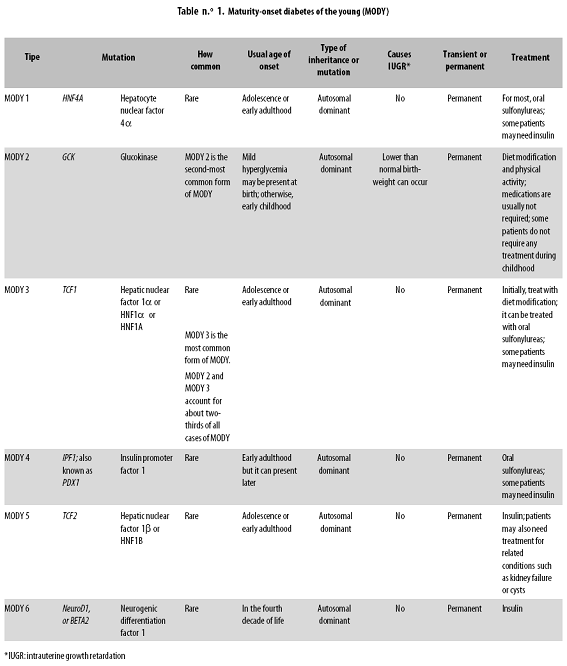
Maturity–Onset Diabetes of the Young (MODY) affects 1– 5% of people with diabetes in the USA and other industrialized countries.1–3 This type of diabetes very often goes unrecognized. The three main features of MODY include: Development of diabetes before the age of 25 to 30 years in absence of pancreatic antibodies, autosomal dominant inheritance (present in at least two family generations), and evidence of residual insulin secretion, that allows for treatment of this condition most of the time with diet or hypoglycemic agents and does not always require insulin therapy. The table depicts the six types of MODY and their characteristics.
We will focus on the management of MODY2 (GCK mutation) and 3 (HNF1–a mutation), which are the most common subtypes, accounting for more than 70% of cases.4–7
Good evidence regarding effects and prognosis of various treatments is still limited for this increasingly recognized cause of diabetes. Although obesity and insulin resistance are not typical features of MODY in comparison with type 2 diabetes mellitus (T2DM); leading an active lifestyle and maintaining a normal weight are essential recommendations for all MODY patients. Weight loss will improve insulin resistance as well as glucose tolerance in obese patients with MODY.8 Sulfonylureas (SUs) remain the medication of first choice in children and adults when dietary therapy is insufficient to maintain normoglycemia.9–11 The use of metiglinides has been implemented in some patients to control post–prandial hyperglycemia.12 Although MODY1, 3, and 4 usually respond very well to oral SUs, due to progressive β–cell failure, a significant proportion of MODY1 and MODY3 patients may eventually require insulin therapy.13 Mutations in the Hepatic nuclear factor 1 alpha gene (HNF1–a) result in MODY3, the most common subtype, which accounts for 8 to 63% of MODY cases.4,5,6 Patients with MODY 3 have a progressive deterioration in
glycemic control and are at risk for microvascular and macrovascular complications.14 Pancreatic exocrine dysfunction has been reported in 12% of adult patients.15 These patients will need pancreatic supplements. Affected individuals usually present with severe hyperglycemia after puberty, which can be treated initially with diet. However, post prandial hyperglycemia will be present in the course of the time due to insufficient insulin production.16 At this point, most patients will need pharmacological treatment.
Compared to other types of diabetes, MODY3 patients are extremely sensitive to the hypoglycemic effect of SUs.17 To avoid hypoglycemia, the initial dose in children should be low (approximately ¼ of the usual starting dose in adults).9,18 Successful management of hyperglycemia with administration of SUs for decades has been reported. 9 HbA1c levels should be repeated at 3 month intervals. If this regimen fails to control blood glucose levels as indicated by a rise in HbA1c, the use of a long acting insulin alone or in combination with SUs should be considered. Insulin is the treatment of choice in pregnant women with MODY3 especially if there is excess in fetal growth.19
Patients with heterozygous Glucokinase (GCK) gene mutations (MODY2) have sustained lifelong mild asymptomatic hyperglycemia, very often present from birth. HbA1c is typically on the upper normal limit. Microvascular and macrovascular complications are rarely developed even in untreated patients.20,21 It is very rare for patients with mutations in the GCK gene to have symptomatic hyperglycemia or to need treatment other than diet. There is very little evidence of clinical benefit with pharmacological therapy.22
BIBLIOGRAPHIC REFERENCES
1. Ledermann HM. Maturity–onset diabetes of the young (MODY) at least 10 times more common in Europe than previously assumed. Diabetologia 1995: 38: 1482. [ Links ]
2. Froguel P, Zouali H, Vionnnet N, Velho G, Vaxillaire M, Sun F, et al. Familial hyperglycemia due to mutations in glucokinase: definition of a subtype of diabetes mellitus. N Eng J Med 1993; 328: 697–702 [ Links ]
3. Gat–Yablonski G, Shalitin S, Phllip M. Maturity onset diabetes of the young–review. Pediatr Endocrinol Rev 2006; 3: 514–20 [ Links ]
4. Chèvre JC, Hani EH, Boutin P, Vaxillaire M, Blanché H, Vionnet H et al. Mutation screening in 18 Caucasian families suggests the existence of other MODY genes. Diabetologia 1998; 41: 1017–1023. [ Links ]
5. Costa A, Bescos M, Velho G, Chevre J, Vidal J, Sesmilo G, et al. Genetic and clinical characterization of maturityonset diabetes of the young in Spanish families. Eur J Endocrinol 2000; 142: 380 –386. [ Links ]
6. Frayling TM, Bulamn MP, Ellard S, Appleton M, Dronsfield MJ, Mackie AD, et al. Mutations in the hepatocyte nuclear factor–1alpha gene are a common cause of maturity–onset diabetes of the young in the U.K. Diabetes 1997; 46: 720–725. [ Links ]
7. Estalella I, Rica I, Perez de Nanclares G, Bilbao JR, Vazquez JA, San Pedro JI, et al. Mutation in GCK and HNF–1 alpha explain the majority of cases with clinical diagnosis of MODY in Spain. Clin Endocrinol 2007; 67: 538–546 [ Links ]
8. Lawson VK, Young RT, Kitabchi AE. Maturity–onset diabetes of the young: an illustrative case for control of diabetes and hormonal normalization with dietary management. Diabetes Care 1981; 4: 108–112 [ Links ]
9. Fajans SS, Brown MB. Administration of sulfonyureas can increase glucose–induced insulin secretion for decades in patients with maturity–onset diabetes of the young (MODY). Diabetes Care 1993; 16: 1254–1261. [ Links ]
10. Shepherd M, Pearson ER, Houghton J, Salt G, Ellard S, Hattersley AT. No deterioration in glycemic control in HNF–1alpha maturity–onset diabetes of the young following transfer from long–term insulin to sulphonylureas. Diabetes Care 2003; 26: 3191–3192. [ Links ]
11. Malecki, MT, Mlynarski W. Monogenic diabetes: implications for therapy of rare types of disease. Diabetes Obes Metab 2008; 10: 607–616. [ Links ]
12. Tuomi T, Honkanen EH, Isomaa B, Sarelin L, Groop LC. Improved prandial glucose control with lower risk of hypoglycemia with nateglinide than with glibenclamide in patients with maturity–onset diabetes of the young type 3. Diabetes Care 2006; 29: 189–194. [ Links ]
13. Herman WH, Fajans SS, Smith MJ, et al. Diminished insulin and glucagon secretory responses to arginine in nondiabetic subjects with a mutation in the hepatocyte nuclear factor4–a/MODY1 gene. Diabetes 1997; 46: 1749–1754. [ Links ]
14. Isomaa B, Henricsson M, Lehto M, Forsblom C, Karanko S, Sarelin L, et al. Chronic diabetic complications in patients with MODY3 diabetes. Diabetologia 1998; 41: 467–473. [ Links ]
15. Vesterhus M, Raeder H, Johansson S, Molven A, Njølstad PR. Pancreatic exocrine dysfunction in maturity–onset diabetes of the young type 3. Diabetes Care, 2008; 31: 306–310 [ Links ]
16. Byrne MM, Sturis J, Menzel S, Yamagata K, Fajans SS, Dronsfield MJ, et al. Altered insulin secretory responses to glucose in diabetic and nondiabetic subjects with mutations in the diabetes susceptibility gene MODY3 on chromosome 12. Diabetes 1996; 45: 1503–1510 [ Links ]
17. Pearson ER, Liddell WG, Shepherd M, Corrall RJ, Hattersley AT. Sensitivity to sulphonylureas in patients with hepatocyte nuclear factor 1 alpha gene mutations: evidence for pharmacogenetics in diabetes. Diab Med 2000; 17: 543–545. [ Links ]
18. Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT. Genetic cause of hyperglycemia and response to treatment in diabetes. Lancet 2003; 362: 1275– 1281. [ Links ]
19. Spyer G, Hattersley AT, Sykes JE, Sturley RH, MacLeod KM. Influence of maternal and fetal glucokinase mutations in gestational diabetes. Am J Obstet Gynecol 2001; 185: 240–241 [ Links ]
20. Velho G, Blanche H, Vaxillaire M, Bellanne–Chantelot C, Pardini VC, Timsit J, et al. Identification of 14 new glucokinase mutations and description of the clinical profile of 42 MODY–2 families. Diabetologia 1997; 40: 217– 224. [ Links ]
21. Segen JV, Bjorkhaug L, Molnes J, et al. Diagnostic screening of MODY2/GCK mutation in the Norwegian MODY Registry. Pediatric Diabetes 2008; 9: 442–449. [ Links ]
22. Murphy R, Ellard S, Hatersley AT. Clinical implications of a molecular genetic classification of monogenic B–cell diabetes. Nat Clin Practice 2008: 4: 200–213. [ Links ]
Recibido: marzo 18 de 2009
Aceptado: marzo 24 de 2009