Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Iatreia
Print version ISSN 0121-0793
Iatreia vol.26 no.3 Medellín July/Sept. 2013
INVESTIGACIÓN ORIGINAL
Polysomnographic evaluation of uninfected babies born to human immunodeficiency virus type 1 positive mothers
Evaluación polisomnográfica de bebés no infectados nacidos de madres VIH-1 positivas
Mario Eduardo Archila Meléndez*1; Margarita María Giraldo Chica*2; William Cornejo Ochoa3; Jorge Alejandro Henao-Mejía4; María Teresa Rugeles López1; Magda Lahorgue Nunes5
1 Grupo Inmunovirología, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia. marioarchila@gmail.com
2 Instituto Neurológico de Antioquia.
3 Grupo Pediaciencias, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia.
4 Department of Immunobiology, Yale University School of Medicine, New Haven, Connecticut, USA.
5 Facultad de Medicina, Pontificia Universidad Católica de Rio Grande do Sul, Brasil.
* La autoría se comparte de manera igualitaria
Recibido: febrero 25 de 2012
Aceptado: junio 25 de 2012
SUMMARY
Introduction: Type 1 human immunodeficiency virus (HIV-1) is a lymphotropic and neurotropic retrovirus. Thus, it causes immunological and neurological alterations particularly in children. In the neonatal period the maturational changes of the central nervous system occur rapidly, and their alteration can be reflected in processes such as the sleep-awake pattern.
Objective: To evaluate sleep organization, EEG and respiratory pattern in newborns to HIV-1 positive mothers.
Methods: 22 infants underwent polysomnography. Delta brushes number in REM and NREM sleep, duration of interburst interval and interhemispheric synchrony were used to calculate EEG maturation. Analysis of the sleep architecture was based on polysomnographic sleep percentage of REM, NREM and transitional sleep to total sleep time.
Results: The difference between electroencephalographically calculated and clinically calculated conceptional age was less than two weeks. Percentages of REM and NREM sleep ranged from 39-64 and 30-58 with a median of 52.5 and 36.5 respectively. Concordance was lower in newborns who had high transitional sleep percentages, compared to that in newborns who did not have high such characteristic (p<0.05).
Discussion: Despite intrauterine exposure to HIV-1 and to antiretroviral drugs we did not observe a significant effect on EEG maturation. The decreased concordance in newborns with high transitional sleep percentages would suggest an alteration in the maturation process, but this aspect itself is not sufficient to consider that intrauterine exposure to HIV-1 and antiretrovirals affect the entire sleep architecture. Future studies should clarify whether the decreased concordance between behavior and NREM sleep is replicable.
KEY WORDS
Central Nervous System, Electroencephalography, HIV; Maternal Exposure, Newborn, Polysomnography, Pregnancy; Sleep
RESUMEN
Introducción: el virus de la inmunodeficiencia humana tipo 1 (VIH-1) es un retrovirus linfotrópico y neurotrópico. Esta característica genera alteraciones inmunológicas y neurológicas particularmente en niños. Durante el período neonatal la maduración del sistema nervioso central ocurre rápidamente, y su alteración puede perturbar diferentes aspectos del desarrollo tales como el ciclo sueño-vigilia.
Objetivo: evaluar la organización del sueño y el patrón electroencefalográfico y respiratorio en recién nacidos VIH-1 negativos hijos de madres VIH-1 positivas.
Métodos: se les hizo polisomnografía a 22 infantes. Se calculó la maduración electroencefalográfica usando el número de ondas delta en sueño REM y NREM, la duración del intervalo interespigas y la sincronía interhemisferica. Se analizó la arquitectura del sueño con base en el porcentaje de sueño REM, NREM y sueño transicional con relación al tiempo total de sueño.
Resultados: la diferencia entre la edad electroencefalográfica y la edad concepcional calculada fue menor de dos semanas. El rango del porcentaje de sueño REM y NREM fue 39-64 y 30-58 y la media fue de 52,5 y 36,5, respectivamente. La concordancia en los recién nacidos con alto porcentaje de sueño transicional fue menor comparada con la de los neonatos con menor porcentaje de sueño transicional (p<0,05).
Discusión: a pesar de la exposición intrauterina al VIH- 1 y a los antirretrovirales, no se evidenciaron cambios significativos en la maduración electroencefalográfica. La disminución de la concordancia en neonatos con alto porcentaje de sueño transicional podría sugerir una alteración en el proceso de maduración, pero este aspecto en particular no es suficiente para considerar que la exposición intrauterina al VIH-1 y a los antirretrovirales afecta toda la arquitectura del sueño. Estudios posteriores deberían aclarar si la disminución entre la concordancia, el comportamiento y el porcentaje de sueño NREM es duplicable.
PALABRAS CLAVE
Electroencefalografía, Exposición Materna, Embarazo, Polisomnografía, Recién Nacido, Sistema Nervioso Central, Sueño, VIH
INTRODUCTION
During neurodevelopment the sleep-wake pattern undergoes important changes. At birth, a newborn at term sleeps from 16 to 18 hours per day, and an additional two hours if the baby is premature. In the neonatal period the sleep-wake cycle has polyphasic characteristics that alternate regardless of the time of day; this is called ultradian rhythm. During the neonatal period maturational changes of the central nervous system occur rapidly. Changes in the sleep pattern are part of this maturation process; during the first year of life the sleep pattern acquires the circadian distribution (1).
Type 1 human immunodeficiency virus (HIV-1) is a lymphotropic and neurotropic retrovirus. Thus, it causes immune and neurological alterations particularly in children (2). The main neurological alterations observed in HIV-1 infected children are progressive encephalopathy characterized by severe developmental delay and affecting cognitive and motor acquisitions, peripheral neuropathy, myelopathy and myopathy, among others (2). The clinical manifestations observed in HIV-1 positive infants with early encephalopathy suggest an alteration in the myelination process that has been confirmed by magnetic resonance imaging findings (3-5).
In adults and infants neurological manifestations are correlated with imaging findings such as atrophy, white matter pallor and basal ganglia mineralization (6,7). An association between imaging findings and high viral loads with poor immunological and clinical status has been described (8). In addition, certain reports indicate a high frequency of severe cerebral atrophy in HIV-1 positive children with early onset of encephalopathies that also show encephalic myelination delay (7) and a correlation between expressive language deficit and imaginological findings (9).
Electroencephalogram (EEG) is a noninvasive procedure that has been recognized as an important tool in the management of newborns with seizures and other signs of neurological diseases as well as in the diagnosis and prognosis of neurological disorders, including encephalopathy (10-12). Polysomnography (PS) enhances the use of EEG particularly in neonates, increasing the information by the evaluation of sleep and respiratory patterns.
No previous studies have been carried out in HIV-1 uninfected children born to HIV-1 positive mothers evaluating the alteration in both sleep and EEG patterns.
The aim of this study was to describe the characteristics of sleep organization, EEG and respiratory patterns in newborns to HIV-1 positive mothers treated with antiretrovirals during pregnancy.
SUBJECTS AND METHODS
Type of study and subjects
This is a descriptive study including 22 infants born to HIV-1 positive mothers who were part of a prospective cohort for the study of neurodevelopment (13). All HIV-1 infected women were under antiretroviral treatment during pregnancy and intravenously at delivery. The neonates received preventive treatment with a single dose of nevirapine 2 mg/kg at 48-72 hours of life plus oral zidovudine 2 mg/kg four times a day during the first six weeks of life.
Laboratory tests and clinical evaluations
To diagnose HIV-1 infection the babies were tested by viral load during the first six months of age. The gestational age (GA), defined as the time elapsed between the first day of the last normal menstrual period and the day of delivery, expressed as completed weeks, was calculated by Capurro's A method (14), and correlated with data obtained during pregnancy controls including obstetric ultrasonography. Conceptional age (CONA) also known as ''chronological age'' or ''postnatal age'' is the time elapsed after birth and in this study is expressed in weeks. Calculated conceptional age (CCONA) also known as ''corrected gestational age'' or ''adjusted age'' was calculated by subtracting the number of weeks the baby was born before the 40th week of gestation from the CONA; in preterm newborns it represents the age of the child from the expected date of delivery (15). A complete physical and neurological evaluation was carried-out by two clinicians in a controlled environment and follow- up was done to finally discard the HIV-1 infection by viral load at 12 months and by ELISA at 18 months of age, respectively.
Polysomnography
Newborns were submitted to one polysomnographic recording between 7 and 41 days of life. The procedure consisted of a continuous record with a digital 32-channel instrument with 11 channels for EEG; the additional channels were for electrooculograms (EOG), submental electromyographies (EMG), electrocardiograms, monitoring of respiratory flow and thoracic movements (16,17). The registry speed was 15 mm/sec and for the EEG the constant time of 0.3 seconds (sec) was used; sensitivity was 10 microvolts per millimeter (µV)/mm) and high frequency filter was of 70 Hz (18). Electrodes were placed according to the international 10-20 system adjusted for newborns (Fp1-C3, C3-O1, Fp1-T3, T3-O1, Fp2-C4-O2, Fp2-T4, T4-O2, C3-Cz and Cz-C4) (17,19,20). The state of the newborn and all movements during the test were recorded and video-registered. All registers were during daytime in supine position and lasted at least 58 minutes (min), and the registry was taken until spontaneous awakening, giving all children the time to have a complete ultradian sleep cycle (i.e. complete registry of rapid eye movement sleep, transitional sleep and non-rapid eye movement sleep).
The electroencephalographic conceptional age was calculated with the following quantitative parameters: number of delta brushes, percentage of inter-hemispheric synchrony, concordance between behavior and EEG and duration of interburst intervals (21).
The recognition of transition from waking to sleeping was made in each newborn using polygraphic (EEG, EOG, EMG and respiratory rhythm) and behavioral (closed and opened eyes, crying and body movements) characteristics (22).
The different states of sleep were determined as follows. i) Rapid eye movement sleep (REM): EEG with continuous mixed activity including theta, delta, alpha and beta waves between 40-80 µV of amplitude, slow and rapid bursts and isolated eye movements in EOG, low amplitudes superimposed with twitches and phasic jerky movements in EMG and variable beat to beat intervals in ECG. ii) Non-rapid eye movement sleep (NREM): EEG continuous 50-150 µV delta waves or tracé alternant defined as 3-8 sec bursts of high amplitude slow waves separated by 4-10 sec low voltage mixed EEG, no eye movements or infrequent eye movements in EOG, low amplitude signals in EMG, predominantly regular rates on ECG, and predominantly regular respiration. iii) Transitional sleep or undetermined sleep: it was defined as a state that does not meet the criteria for REM or NREM sleep (23), the acceptable percentages of REM and NREM sleep were not established a priori.
For respiratory patterns we placed a nasal transducer, an abdominal band two centimeters above the umbilicus and a pulse oxymeter in the second finger of the right hand. Apnea was divided into central obstructive and mixed and was measured in each sleep stage. Periodic breathing was defined as three or more apneas lasting more than three sec with intervals between breaks of less than 20 sec (24); it was quantified in relationship with total sleep time (TST) in each patient.
The total number of delta brushes, frontal and temporal sharp transients, the presence of delta frontal rhythmic activity, concordance between behavioral sleep patterns and EEG, degree of interhemispheric synchrony, and measurement of interburst interval were determined during one 5 min epoch of REM and of NREM sleep (the most typical epochs in each PS were selected). These events were defined as follows: (a) Delta brushes: described by Lombroso (25) as a spindle of varying frequencies (8-22 Hz) associated with a delta wave, were scored in one channel (T3- O1 or T4-O2) during each examination in REM and NREM sleep. (b) Temporal sharp transients: we verified the presence/absence of isolated sharp waves in the temporal region during one five min epoch of REM and NREM sleep (26,27). (c) Frontal sharp transients: biphasic negative-positive sharp waves, with maximum amplitude in the prefrontal regions and often followed by a slow wave (28,29) were verified during one five min epoch of REM and NREM sleep. (d) Delta frontal rhythmic activity (anterior slow dysrhythmia): bursts of polymorphic or monomorphic delta activity in the frontal areas that may follow frontal sharp transients (27). (e) Concordance: as defined by Lombroso (25) is the agreement between behavioral and physiological parameters of REM and NREM sleep. We scored one point for each 5 behavioral parameters (eyes closed, presence or absence of phasic activity, presence or absence of crying, smile and groan, isolated head or body movements and respiration pattern). A score of 5 points indicated concordance of the behavioral state with the physiological pattern (12). (f) Interhemispheric synchrony: the degree of synchrony of interhemispheric activity was measured during 5 consecutive min of NREM sleep (25). The percentage of synchronic bursts was calculated. (g) Interburst interval: the maximum interval duration (defined as having no activity greater than 15 µV in amplitude) between 2 bursts of activity during 5 consecutive min of NREM sleep was measured bilateraly.
Statistical analysis
We used frequency distribution and position (median) measurements for the description; the Student t-test for the comparison of the electroencephalographically calculated conceptional age with the real conceptional age; and the Pearson correlation coefficient for the concordance between behavioral activity and EEG pattern.
The protocol was approved by the Ethics Committee of the Faculty of Medicine, University of Antioquia, and the execution was adjusted according to the international ethics recommendations. Parents signed an informed consent to have their babies included in the study.
RESULTS
Subjects, EEG maturation and clinical evaluations
We evaluated 22 newborns, 9 males (41%), and 13 females (59%). The median of the mother's age was 26 years (range: 19 to 43 years). Median birth gestational age was 38 weeks (range: 34 to 40 weeks). The bioelectric maturation was consistent with conceptional age in all patients, median 40 weeks (range: 36 to 44 weeks). The difference between electroencephalographically calculated and clinically calculated conceptional age was less than 2 weeks, without reaching any significant differences -0.84 p<0.05 (table 1). Only one newborn exhibited an altered physical examination due to cleft lip and palate, but it was included in all analyses. The median of the Apgar score at the first min was 9 (range: 8 to 10) and at five minutes it was 10 (range: 9 to 10). No clinical or neurological disorders were observed during the neonatal period.
Polysomnography
The sleep cycle lasted between 58 and 120 min of continuous sleep (median 89 min, SD: 17.7). The median percentage of REM sleep was 52.5% (range: 39 to 64%), of NREM sleep was 36.5% (range: 30% to 58%) and of transitional sleep was 10% (range: 0% to 17%); the detailed data are presented in table 1.
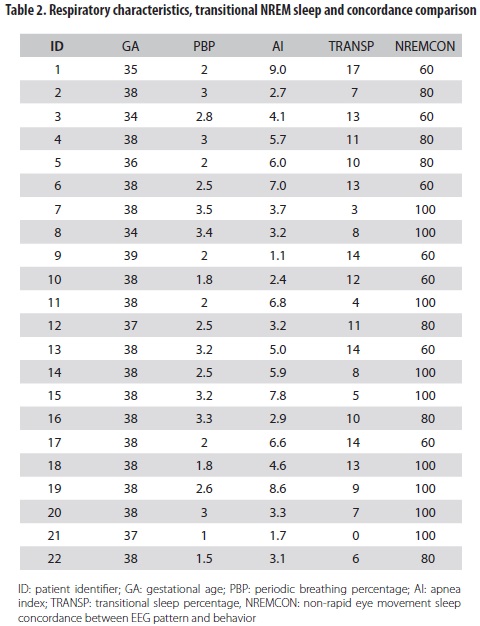
The score of concordance between REM sleep and behavior was 100% for all babies. In NREM sleep it varied from 60% to 100% (median: 81.82, SD: 17.3). Concordance was lower in newborns who had high transitional sleep percentages, compared to concordance in those who did not have such characteristic (correlation -0.59, p<0.05) (table 2).
The periodic breathing percentage was less than 5%; the median duration of central apneas was 8 and 7 sec in REM and NREM sleep respectively. None of the newborns exhibited apneas up to 10 sec of duration and no evidence of obstructive or mixed apneas was recorded; oxygen saturation was between 90% and 100%.
DISCUSSION
The main limitation of the present study is the lack of HIV-1 infected neonates and of healthy newborns to compare with the group of HIV-1-exposed non-infected babies studied here. Therefore, the present findings were compared with those described in the literature on the sleeping and electroencephalographic ontogeny of healthy children or children with conditions different from HIV-1 exposure, considering that there are no previous reports on a similar cohort.
The polysomnographic recording is a useful tool to evaluate sleep ontogeny in neonates and also the impact of clinical and neurological disorders on the developing brain (30).
The aim of this study was to describe the possible impact of intrauterine HIV-1 or antiretroviral exposure on sleep organization, EEG maturation and respiratory patterns. However, analysis of these three elements suggests that these intrauterine exposures do not significantly affect the bioelectrical maturation process in children that have not become infected themselves.
Sleep-wake cycle maturation precedes the myelination of the majority of the prosencephalus, suggesting that myelination does not have high relevance in this process. The sleep-wake cycle for the term neonate has a mean duration of 50 min (range 30 to 70 min). This duration tends to increase with conceptional age (31-34). In this study the median sleep-wake cycle was 89 min (range 58 to 120 min). It is important to specify that the technician did not awake the babies after the first cycle; she allowed them to wake-up spontaneously.
In the term neonates, the majority of apneas are central (35). Several studies have shown that obstructive or mixed apneas are rare in healthy neonates (36). In this study all apneas were central and none of them lasted more than 10 sec or were accompanied by a drop in oxygen saturation or bradycardia. In addition, the proportion of periodic respiration was less than 5% of the total sleep time in all neonates, indicating a mature respiratory pattern. Again, these results suggest that intrauterine HIV-1 or antiretroviral exposure do not affect the respiratory pattern.
REM sleep predominates during the neonatal period; it constitutes around 60% to 65% in the term neonates and starts decreasing with time until it reaches adult levels of around 25% in the complete cycle (37).
The NREM sleep percentage increase from birth to the end of the first month of life, reaching at this time around 50%-55% of each cycle (37,38). In this study the percentages of REM and NREM sleep were in line with the values previously described in the literature.
Transitional sleep is an intermediate period between the REM and NREM phases and exhibits characteristics of both. The normal amount of transitional sleep has not been clearly determined. In one study this percentage, in relation with the conceptional age, ranged between 31.4% in neonates below 34 weeks to 7.2% at 40 weeks (39). Nunes et al. reported percentages between 19% and 11% in full-term infants (40,41). There are no studies indicating if there is a correlation between abnormalities in the transitional sleep and neurological pathologies. The percentages found in our study range up to 10%.
Lombroso and collaborators found that concordance between behavioral and both REM and NREM sleep was 100% at 38 weeks of conceptional age in contrast with the findings in the present cohort in which we found a subgroup with lower proportion in NREM sleep mainly the ones with higher transitional sleep percentages. Concordance is considered as part of the cerebral maturation parameters. These findings might suggest that those newborns have a behavioral pattern less immature than the expected one for the conceptional age (25).
In summary, sleep architecture and electroencephalographic and respiratory patterns were normal in all babies born to HIV-1 positive mothers and exposed perinatally to antiretroviral therapy.
The cortical maturation process is a complex and prolonged event that begins at the end of the first gestational trimester and continues after the baby is born. It seems to be intimately related to variations of the neonatal electroencephalographic patterns.
The decrease of behavioral concordance in NREM sleep in these newborns with high transitional sleep percentage might suggest an alteration in the maturation process but this aspect alone is not sufficient to consider that HIV-1 and antiretrovirals intrauterine exposure affect the entire sleep architecture.
The results of this descriptive study support the need for a better understanding of the sleep maturation process and the possible effects of environmental and pharmacological variables. Future studies including a group of healthy newborns may clarify the differences in sleep and respiratory patterns between the two groups.
REFERENCIAS BIBLIOGRÁFICAS
1. Shimada M, Takahashi K, Segawa M, Higurashi M, Samejim M, Horiuchi K. Emerging and entraining patterns of the sleep-wake rhythm in preterm and term infants. Brain Dev. 1999 Oct;21(7):468–73. [ Links ]
2. Orejón de Luna G, Mateos F, Simón de las Heras R, Martínez Menéndez B, Ramos Amador JT, Muñoz González A. [Neurological impairment in children with HIV infection]. Rev Neurol. 1996 Mar;24(127):278–84. [ Links ]
3. Tardieu M, Le Chenadec J, Persoz A, Meyer L, Blanche S, Mayaux MJ. HIV-1-related encephalopathy in infants compared with children and adults. French Pediatric HIV Infection Study and the SEROCO Group. Neurology. 2000 Mar 14;54(5):1089–95. [ Links ]
4. Czornyj LA. [Encephalopathy in children infected by vertically transmitted human immunodeficiency virus]. Rev Neurol. 2006;42(12):743–53. [ Links ]
5. Rotta NT, Silva C, Ohlweiler L, Lago I, Cabral R, Gonçalves F, et al. [Aids neurologic manifestations in childhood]. Rev Neurol. 1999;29(4):319–22. [ Links ]
6. Sánchez-Ramón S, Bellón JM, Resino S, Cantó-Nogués C, Gurbindo D, Ramos J-T, et al. Low blood CD8+ T-lymphocytes and high circulating monocytes are predictors of HIV-1-associated progressive encephalopathy in children. Pediatrics. 2003 Mar;111(2):E168–75. [ Links ]
7. Johann-Liang R, Lin K, Cervia J, Stavola J, Noel G. Neuroimaging findings in children perinatally infected with the human immunodeficiency virus. Pediatr Infect Dis J. 1998 Aug;17(8):753–4. [ Links ]
8. Wolters PL, Brouwers P, Moss HA, Pizzo PA. Differential receptive and expressive language functioning of children with symptomatic HIV disease and relation to CT scan brain abnormalities. Pediatrics. 1995 Jan;95(1):112–9. [ Links ]
9. Depas G, Chiron C, Tardieu M, Nuttin C, Blanche S, Raynaud C, et al. Functional brain imaging in HIV- 1-infected children born to seropositive mothers. J Nucl Med. 1995 Dec;36(12):2169–74. [ Links ]
10. Lombroso CT. Neonatal polygraphy in full-term and premature infants: a review of normal and abnormal findings. J Clin Neurophysiol. 1985 May;2(2):105–55. [ Links ]
11. Scher MS, Barmada MA. Estimation of gestational age by electrographic, clinical, and anatomic criteria. Pediatr Neurol. 1987;3(5):256–62. [ Links ]
12. Nunes ML, Da Costa JC, Moura-Ribeiro M V. Polysomnographic quantification of bioelectrical maturation in preterm and fullterm newborns at matched conceptional ages. Electroencephalogr Clin Neurophysiol. 1997 Mar;102(3):186–91. [ Links ]
13. Gómez C, Archila ME, Rugeles C, Carrizosa J, Rugeles MT, Cornejo JW. [A prospective study of neurodevelopment of uninfected children born to human immunodeficiency virus type 1 positive mothers]. Rev Neurol. 2009;48(6):287–91. [ Links ]
14. Goyal SC, Tak SK, Bhandari B. Determination of gestational age: comparative accuracy of different methods. Indian J Pediatr. 1989;56(1):115–9. [ Links ]
15. Rossavik IK, Fishburne JI. Conceptional age, menstrual age, and ultrasound age: a second-trimester comparison of pregnancies of known conception date with pregnancies dated from the last menstrual period. Obstet Gynecol. 1989 Mar;73(2):243–9. [ Links ]
16. Holmes GL, Lombroso CT. Prognostic value of background patterns in the neonatal EEG. J Clin Neurophysiol. 1993 Jul;10(3):323–52. [ Links ]
17. Anders T, Emde R, Parmelee A. A manual of standardized terminology, techniques and criteria for scoring of states of sleep and wakefulness in newborn infants. UCLA Brain Information Service, editor. Los Ángeles: UCLA; 1971. [ Links ]
18. da Costa JC. O sono em recém-nascidos: aspectos polissonográficos. In: Reimão R, editor. Sono aspectos atuais. São Paulo: Atheneu; 1990. p. 133–61. [ Links ]
19. Lombroso C. Neonatal EEG poligraphy in normal and abnormal newborns. In: Niedermeyer E, editor. Electroencephalography: basic principles, clinical applications and related fields. 3rd ed. London: Williams & Wilkins; 1993. p. 803–75. [ Links ]
20. Nunes M, Da Costa J, Roitman I, Fernandez R. Guia técnico para execução de registro poligráfico e eletroencefalograma no período neonatal. J. epilepsy clin. neurophysiol. 1996;2(1):15. [ Links ]
21. Nunes ML, da Costa JC, Taufer L, da Silveira CM. [Value of EEG in the characterization and prognosis of neurological diseases in premature infants]. Arq Neuropsiquiatr. 1995 Oct;53(3-B):625–30. [ Links ]
22. Curzi-Dascalova L, Monod N, Guidasci S, Korn G. [Waking-sleeping transition in the newborn baby and in infants before the age of 3 months (author's transl)]. Rev Electroencephalogr Neurophysiol Clin. 1981 Oct;11(1):1–10. [ Links ]
23. Mirmiran M, Maas YGH, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev. 2003 Aug;7(4):321–34. [ Links ]
24. Kelly DH, Shannon DC. Periodic breathing in infants with near-miss sudden infant death syndrome. Pediatrics. 1979 Mar;63(3):355–60. [ Links ]
25. Lombroso CT. Quantified electrographic scales on 10 pre-term healthy newborns followed up to 40- 43 weeks of conceptional age by serial polygraphic recordings. Electroencephalogr Clin Neurophysiol. 1979 May;46(4):460–74. [ Links ]
26. Hughes JR, Fino JJ, Hart LA. Premature temporal theta (PT theta). Electroencephalogr Clin Neurophysiol. 1987 Jul;67(1):7–15. [ Links ]
27. Stockard-Pope J, Werner S, Bickford R. Atlas of neonatal electroencephalography. 2nd ed. Raven Press; 1992. [ Links ]
28. Monod N, Dreyfus-Brisac C, Ducas P, Mayer M. [The EEG of the newborn infant at term. Comparative study in the newborn infant in cephalic presentation and breech presentation]. Rev Prat. 1960 May;102:375–9. [ Links ]
29. Arfel G, Leonardon N, Moussalli F. [Density and dynamic of frontal sharp waves (encoches pointues frontales) during sleep in new-borns and infants (author's transl)]. Rev Electroencephalogr Neurophysiol Clin. 1977;7(3):351–60. [ Links ]
30. Arfel G, Leonardon N, Moussalli F. [Density and dynamic of frontal sharp waves (encoches pointues frontales) during sleep in new-borns and infants (author's transl)]. Rev Electroencephalogr Neurophysiol Clin. 1977;7(3):351–60. [ Links ]
31. Purpura D, Shoffer R. Principles of synaptogenesis and their applications to ontogenetic studies of mammalian cortex. In: Clemente C, Purpura D, Mayer F, editors. Sleep and the maturing nervous system. New York: Academic Press; 1972. p. 3–32. [ Links ]
32. Louis J, Cannard C, Bastuji H, Challamel MJ. Sleep ontogenesis revisited: a longitudinal 24-hour home polygraphic study on 15 normal infants during the first two years of life. Sleep. 1997 May;20(5):323–33. [ Links ]
33. Salzarulo P, Fagioli I, Salomon F, Ricour C, Raimbault G, Ambrosi S, et al. Sleep patterns in infants under continuous feeding from birth. Electroencephalogr Clin Neurophysiol. 1980 Aug;49(3-4):330–6. [ Links ]
34. Curzi-Dascalova I, Mirmiran M. Manual of methods for recording and analysing sleep-wakefulness states in preterm and full-term infants. Paris: INSERM; 1996. [ Links ]
35. Guilleminault C, Ariagno R, Korobkin R, Nagel L, Baldwin R, Coons S, et al. Mixed and obstructive sleep apnea and near miss for sudden infant death syndrome: 2. Comparison of near miss and normal control infants by age. Pediatrics. 1979 Dec;64(6):882–91. [ Links ]
36. Flores-Guevara R, Plouin P, Curzi-Dascalova L, Radvanyi MF, Guidasci S, Pajot N, et al. Sleep apneas in normal neonates and infants during the first 3 months of life. Neuropediatrics. 1982 May;13 Suppl:21–8. [ Links ]
37. Ohayon MM, Carskadon MA, Guilleminault C, Vitiello M V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004 Nov 1;27(7):1255–73. [ Links ]
38. Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006 Mar;117(3):741–53. [ Links ]
39. Curzi-Dascalova L, Peirano P, Morel-Kahn F. Development of sleep states in normal premature and full-term newborns. Dev Psychobiol. 1988 Jul;21(5):431–44. [ Links ]
40. Nunes M, Da Costa J. Manual de EEG e polissonografia neonatal: atlas de tracados. Porto Alegre: EDPUCRS; 2003. [ Links ]
41. Scher MS, Steppe DA, Dahl RE, Asthana S, Guthrie RD. Comparison of EEG sleep measures in healthy full-term and preterm infants at matched conceptional ages. Sleep. 1992 Oct;15(5):442–8. [ Links ]