Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Iatreia
Print version ISSN 0121-0793
Iatreia vol.27 no.2 Medellín Apr./June 2014
ORIGINAL RESEARCH
Development of pediatric hydronephrosis patients visiting the San Vicente Foundation University Hospital, Medellín, Colombia
Paulina Vélez-Tejada1; Laura Niño-Serna1; Lina María Serna-Higuita2,4; Ana Katherina Serrano-Gayubo2, Catalina Vélez-Echeverri2,4; Juan José Vanegas-Ruiz2,4; Javier Mauricio Sierra Abaúnza3; Vilma Piedrahíta-Echeverry2,1
1 Pediatrics Resident. School of Medicine, University of Antioquia, Medellín, Colombia.
2 Pediatric Nephrologist, Hospital Pablo Tobón Uribe, Medellín, Colombia.
3 Clinical Epidemiologist. University of Antioquia, Medellín, Colombia.
4 Pediatric Nephrologist, Department of Pediatrics and Childcare, School of Medicine, University of Antioquia, Medellín, Colombia.
ABSTRACT
Hydronephrosis is one of the most common congenital malformations detected on prenatal ultrasounds. Moderate and severe cases are often associated with urological abnormality.
OBJECTIVE
To describe a series of pediatric patients diagnosed with hydronephrosis determining their etiology, prenatal diagnosis and frequency of chronic kidney disease (CKD).
MATERIALS AND METHODS
A descriptive, retrospective study.
RESULTS
The records of 924 patients between the ages of 0 and 18 years were evaluated, 35.7% female and 64.3% male. In 14.4% (133) the diagnosis was prenatal. Hydronephrosis was bilateral in 198 patients (28.5%). In 18.3% (169) no associated urological abnormality was found, reaching 4.2% in CKD (7). Ureteropelvic stenosis was diagnosed in 23.3% (216) followed with 21.5% VUR (199) and posterior urethral valves in 9.4% (87), reaching 10.2% ERC (93). When the hydroneprhosis was diagnosed by urography, those patients presented 11.3% of chronic kidney disease vs. 8.4% in whom the diagnosis was made by ultrasound, when the hydronephosis diagnosed was by prenatal vs postnatal ultrasound, the percentage of CKD was 4.8% vs 10.8%, respectively.
CONCLUSION
Early diagnosis of hydronephrosis allows the detection of urologic abnormalities susceptible of treatment. Although there are still many questions about which one is the ideal strategy of follow up; the ultrasonography, voiding cystourethrogram, urography, scintigraphy and magnetic resonance urography in selected patients are the most useful tools in order to evaluate urinary tract anomaly.
KEY WORDS
Hydronephrosis, Prenatal Diagnosis, Ultrasonography, Vesico-Ureteral Reflux, Ureteral Obstruction
INTRODUCTION
Hydronephrosis, a term used to describe dilation of the renal collecting system, is one of the most commonly detected congenital malformations on prenatal ultrasounds (1); its prevalence varies between 1% and 5% in fetuses (2,3) and may reach 0.5% in newborns (4). Different classification systems have been proposed to assess its severity, usually based on the anteroposterior diameter of the renal pelvis measured by ultrasound, which is considered normal when it is below 7 mm (2,5). In 1993, the Society for Fetal Urology developed a classification system that assesses the grade of dilation based on the sonographic appearance of the renal parenchyma and collecting system with a score that ranges between 0 and 4 (1,6). Moderate and severe cases with diameters greater than 15 mm (grades 3 and 4) are often associated with ureteropelvic junction obstruction, posterior urethral valves, and vesicoureteral reflux (VUR); mild cases (grades 1 and 2) mostly resolve spontaneously (7). Approximately 30% to 40% of prenatally diagnosed hydronephrosis cases persist in the postnatal stage, between 30% and 40% resolve spontaneously during the first two years of life (7,8), and less than 15% require surgery (4).
Today, hydronephrosis remains controversial (9). It could be suspected that a greater degree of hydronephrosis is correlated with severity of the underlying cause, but this hypothesis is debatable, particularly in patients with VUR, for which some studies have shown its presence even in mild cases of hydronephrosis (5). Moreover, there is no good correlation between the hydronephrosis grade and the VUR severity (10). Hwang et al conducted a cohort study in which they observed that employing a voiding cystogram (VCUG) to evaluate prenatal hydronephrosis leads to detection of VUR in 9% to 21% of cases (10). VUR is an important abnormality because it is associated with recurrent urinary tract infections (UTIs) and renal damage (10); thus, proper evaluation and postnatal follow-up are important for differentiating which patients are at risk for renal parenchymal damage and which are not (1).
Due to the clinical significance and potential risk for long-term complications, such as UTIs and chronic renal failure (11), this study aimed to describe pediatric hydronephrosis patients who visited the San Vicente Foundation University Hospital (HUSVF, in Spanish) of Medellín, Columbia, between 1960 and 2010. The investigation sought to determine the causes of hydronephrosis and the frequency of prenatal diagnosis of chronic kidney disease (CKD) in the studied population.
MATERIALS AND METHODS
A descriptive, retrospective study was conducted in which records of patients who attended the outpatient Pediatric Nephrology Clinic of the HUSVF with a hydronephrosis diagnosis during the years 1960-2010 were reviewed. All of the subjects underwent VCUG and other imaging studies, based on the findings and historic timepoint of the diagnosis. These studies included excretory urography, renal ultrasound (within the previous three decades), and renal scintigraphy (within the previous two decades).
The evaluated patients were divided into two groups according to the imaging studies used for the initial diagnosis of hydronephrosis; the first group included patients whose diagnosis was made by excretory urography, and the second group included patients whose initial exam was a renal ultrasound. This grouping was necessary because of the different historical periods included in this study: Colombia adopted the use of ultrasound beginning in the eighties; previously, excretory urography was the only available imaging resource for diagnosing hydronephrosis.
Variables that were considered for analysis were sex, time of diagnosis (prenatal or postnatal), type of hydronephrosis (unilateral or bilateral), and presence or absence of anatomical malformations of the urinary tract. For the diagnosis of VUR, patients with the following associated clinical conditions were excluded: valves, neurogenic bladder, ureterocele, etc. The development of CKD was also evaluated, which was defined as increased creatinine values above the normal age range for greater than three months that did not decrease during the follow-up.
One of the authors (VP) has been responsible for the initial management and the clinical and statistical follow-up of patients included in this study from the first year of the cohort, which includes individuals under 18 years old attending the Pediatric Nephrology Clinic of the HUSVF between January 1, 1960, and December 31, 2010.
A data recollection format was utilized from the clinical history, which was typed into Microsoft Excel and subsequently analyzed using SPSS v.17.O. Standards for the ethical aspects of research involving human subjects, as set out in Resolution 8430 of 1994 of the Ministry of Health of Colombia, were followed. Confidentiality of the personal data was preserved.
RESULTS
In total, there were 924 eligible clinical records of patients under 18 years. The sex distribution was 594 men (64.3%) and 330 (35.7%) women (male/female ratio: 1.8:1). In 133 patients (14.4%), the diagnosis was made prenatally; in the other 791 (85.6%) patients, the diagnosis was postnatal. Of the 694 patients with information on the type of hydronephrosis, 198 were bilateral (28.5%), and 496 (71.5%) were unilateral. In the remaining 230 patients, there were no data on the type of hydronephrosis (unilateral or bilateral).
Of the 198 patients diagnosed with bilateral hydronephrosis (data from 694 patients), the most common causes were as follows: 25% VUR (50 patients), 20.7% posterior urethral valves (41 patients), 0.1% ureteropelvic stenosis (18 patients), and 19.2% normal (38 patients). Patients without associated abnormalities: This group consisted of 169 of the 924 patients (18.3%), of whom 108 were men (63.9%) and 61 (36.1%) were women (male/female ratio 1.8:1). Follow-up was performed on 164 patients in the outpatient clinic, and it was found that seven of them (4.3%) developed CKD. Patients with urinary tract abnormalities: In total, there were 755 (81.7%) patients, including 486 men (64.4%) and 269 women (35.6%) (male/female ratio: 1.8:1). The most frequent abnormality was ureteropelvic junction obstruction [with 219 patients (28.6%)] followed by VUR [with 199 patients (26.4%)] (Table 1).
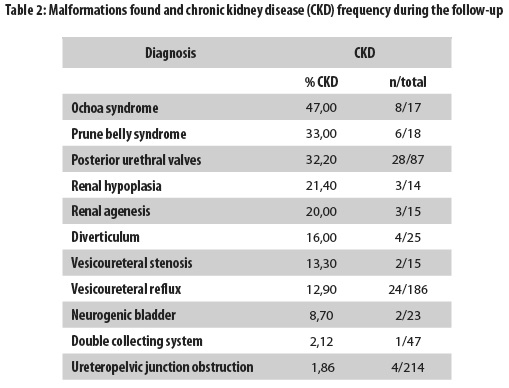
Neurogenic bladder was diagnosed in 23 of the 755 patients (3.0%), and in 21 of these patients, the condition was secondary to myelomeningocele; the sex distribution was 13 women (56.5%) and 10 men (43.5%). During the follow-up, CKD was found in two of these 23 patients (8.7%) and was present in eight (47%) of the 17 patients with Ochoa syndrome (inversion of facial expression, voiding dysfunction, and constipation) (Table 2). It was possible to track 907 of the patients, and CKD was diagnosed in 93 of them (10.2%). Table 2 shows that the most commonly observed abnormalities associated with CKD development were Ochoa syndrome, renal hypoplasia, prune belly syndrome, and posterior urethral valves. Of the patients with ureteropelvic junction obstruction, which was the most commonly found abnormality in this series of patients, only four of 214 (1.9%) progressed to CKD (Table 2).
Another 75 patients of the entire 907 subjects who were tracked had other conditions (ureterocele: eight cases; stones: seven cases; cystic dysplasia: 43 cases; megaureter: 15 cases; and Wilms' tumor: two cases) that did not progress to CKD.
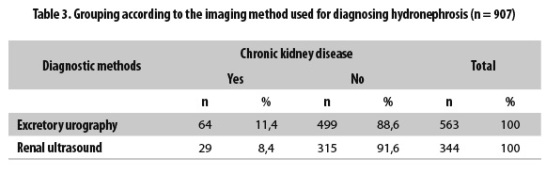
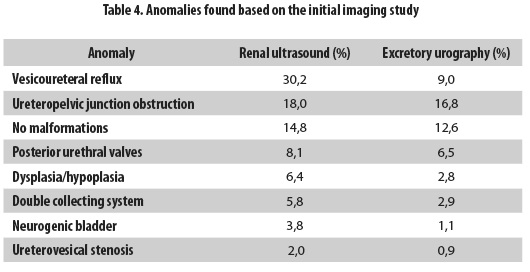
Nine hundred and seven patients were grouped with follow-up according to the diagnostic method used for detecting hydronephrosis; the first group included the 563 patients whose diagnosis was made by excretory urography, and the second group included the 344 patients who were diagnosed by renal ultrasound. CKD was observed during the follow-up in 64 patients from the first group (11.4%) and in 29 (8.4%) patients from the second (Table 3). The anomalies found according to the initial imaging study are presented in Table 4.
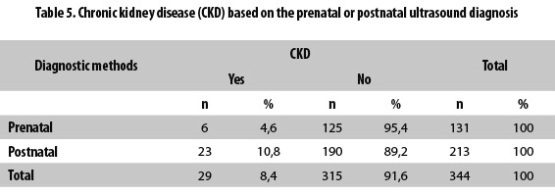
CKD was found in six (4.6%) of the 131 patients with a prenatal ultrasound diagnosis of hydronephrosis and in 23 (10.8%) of the 213 patients whose ultrasound diagnosis was postnatal (Table 5).
In 43 patients (4.6%), other anorectal and genital malformations were found, namely, imperforate anus in 26 patients, hypospadia in four patients, cloaca in four patients, bladder hypoplasia in three patients, urachus in two patients, ambiguous genitalia in one patient, VACTERL association in one patient, and bladder exstrophy in one patient.
DISCUSSION
Hydronephrosis is the most commonly detected anomaly by prenatal ultrasound (4,12). It can be caused by many urological conditions, but in 50% to 70% of cases, no anatomical or functional alteration is found, and they resolve spontaneously (11). Only a small number of patients, especially those with severe hydronephrosis, need surgery (6,13). There are no predictive markers that determine the most appropriate treatment (13); the following are some of the parameters used to define the requirement for surgery: hydronephrotic kidney function of less than 40% compared with healthy kidneys (14), impaired differential function greater than 5% at followup, increasing hydronephrosis, severe hydronephrosis in a single kidney, and recurrent UTIs (6,15).
The biggest challenge in the postnatal management of hydronephrosis is distinguishing which patient is at risk for renal failure to minimize the use of unbeneficial invasive studies (1,8). Various imaging modalities exist. Renal ultrasound is a low-cost, noninvasive method that is free of irradiation but only provides anatomical information; in addition, its results may be altered according to the radiologist's experience and the patient's hydration status (11). A VCUG provides more anatomic details, particularly of the bladder and urethra, but its disadvantages are radiation, the requirement for vesical catheterization, and a susceptibility to UTIs (10,11). Scintigraphy, using either diethylenetriamine pentaacetic acid labeled with technetium 99m (DTPA) or mercaptoacetyltriglycine marked with technetium 99m (MAG3) (diuretic renogram), is used when obstruction is the suspected cause of a dilated renal pelvis; in addition, this technique calculates the differential renal function (11, 16).
Seventy percent to 90% of patients with severe hydronephrosis may have significant urological disease. Thus, it is clear that monitoring requires imaging studies such as renal ultrasound, VCUG, and, based on the findings, renal scintigraphy—as well as (more recently) functional MR urography (17). However, there is controversy regarding patients diagnosed with mild hydronephrosis; some authors report a benign course, but others report the progression of hydronephrosis and the frequent need for surgery. Many studies have shown that a normal ultrasound during the first week of life is not sufficient to rule out malformations or abnormalities of the urinary tract given that these conditions are not initially detected in up to 15% of patients (11). In a study of 213 children with mild to moderate hydronephrosis, there was a 39% significantly obstructive uropathy (ureteropelvic junction obstruction, vesicoureteral stenosis, and posterior urethral valves) (11). Another study found that 24% of children diagnosed with hydronephrosis require surgery; by contrast, some authors have observed that in more than 95% of children with mild hydronephrosis, resolution occurs without recurrence (11). Considering that ultrasound is a noninvasive method, our first proposal is to continue using ultrasound monitoring to confirm the improvement or worsening of hydronephrosis (11). The first renal ultrasound should be performed after the fifth day of life, when the diuresis and hydration statuses are adequate, and the second ultrasound should be performed at four to six weeks of age (18); a renal ultrasound is also recommended at two years of age to determine if there is complete recovery.
Another existing controversy is whether a VCUG should be performed on all patients diagnosed with hydronephrosis; previous studies have shown that VCUG can detect VUR in 9% to 21% of cases (11). VUR in this study represented 26.4% of the anomalies detected, a proportion that is especially significant because high grade VUR is associated with renal scarring (19), arterial hypertension, and CKD (18). Gokce et al, in a study of 256 children referred for the prenatal diagnosis of hydronephrosis, found that hydronephrosis, urinary infections, and VUR were independent risk factors for renal parenchymal lesions. By contrast, up to 27% of patients with a history of prenatal hydronephrosis and grades 2-5 VUR can have a normal postnatal ultrasound (10,20,21); consequently, some groups recommend a VCUG in all children with prenatal hydronephrosis, regardless of the postnatal findings (11,12).
Other authors have questioned the clinical significance of hydronephrosis and suggest resorting to VCUG when the patient has ultrasound findings consistent with renal dysplasia or hypoplasia, renal cystic disease, cortical thinning, loss of corticomedullary differentiation, high-grade unilateral or bilateral dilation, alteration in the kidney number or kidney size, presence of ureteroceles or hydroureteronephrosis, or suspected bladder outlet obstruction (such as thickening of the bladder wall or ureters) (1,6,15). Other ultrasound findings that are indications for a VCUG are the presence of caliectasis and an anteroposterior pelvic diameter greater than 15 mm (6,11). Regarding this situation, the Colombian health system does not allow adequate access to health services, especially for the pediatric population, leading to delayed diagnosis and irreversible consequences. Consequently, the second proposal of this pediatric nephrology group is to perform a VCUG in all patients with hydronephrosis (defined as a renal pelvis AP diameter greater than 7 mm) that does not improve sonographically—with the aim of detecting VUR or urethral abnormalities. If the VCUG is normal and there is increased dilation of the renal pelvis during the follow-up, a diuretic renogram with DTPA or MAG3 or an excretory urography should be performed, depending on the patient's age and renal maturity. In patients diagnosed with bilateral hydronephrosis, a VCUG should be performed during the first postnatal week (15) because these individuals have a higher risk of abnormalities, such as posterior urethral valves, prune belly syndrome, and urethral atresia, which are diseases with high risk of impairing renal function, especially when the diagnosis is delayed.
In this study, similar to the previous investigations, ureteropelvic junction obstruction was the main cause of hydronephrosis (14); this condition was found in 28.6% of patients (10,13,22), followed by VUR (26.4%), posterior urethral valves (11.5%), and, less often, other abnormalities. The female/male ratio was 1.8:1, similar to that found in other studies, such as the investigation by Asli et al (23). As a major finding, 169 patients (18.3%) did not have any anatomical or functional abnormalities during the follow-up; these data differ from previous studies in which transient hydronephrosis without clinical sequelae may represent 80% of cases (11). One proposed explanation is that the institution where this study was conducted is a hospital of fourth level complexity, to which highly selected patients come. Nonetheless, our results cannot be compared with those from other studies because the latter covered a historical timeframe in which hydronephrosis was diagnosed very differently from today.
Seven of our patients (4.2%) without anatomical abnormalities developed CKD, possibly caused by another disease that led to a renal impairment or urinary tract malformation not initially observed; unfortunately, we do not have these data. In this series, a low percentage of patients had a prenatal diagnosis of hydronephrosis (14.4%), which can be explained because ultrasound was only available in Colombia beginning in the eighties. Using this method during the prenatal period allows for planning strategies to prevent complications (21), such as episodes of pyelonephritis, hypertension, or even end-stage renal disease (20). In this study, we found that the percentage of CKD cases was lower in patients with a prenatal diagnosis of hydronephrosis than in those with a postnatal diagnosis (4.8% and 10.3%, respectively). Moreover, the detection and classification of the hydronephrosis severity by prenatal ultrasound has prognostic value because up to 99% of patients diagnosed with severe prenatal hydronephrosis have urological disease (21), which may compel a more thorough study of this group of patients. The aforementioned discussion justifies our third proposal that all pregnant women have access to a third-level ultrasound to search for renal malformations.
CONCLUSION
Hydronephrosis, as a casual imaging finding or during the study of other diseases, such as UTI, facilitates the detection of monitorable urological abnormalities or surgical correction. Prenatal diagnosis has enabled early detection to thereby initiate appropriate clinical monitoring and timely intervention, all with the goal of avoiding future complications, such as episodes of pyelonephritis or even end-stage renal disease.
In selected patients, ultrasounds, VCUGs, excretory urography, renograms, and functional MR urography are the most useful diagnostic imaging techniques for evaluating urinary tract dilation. However, there are still many doubts about the ideal strategy for distinguishing the child who benefits from a complete examination from one who has a benign lesion that does not merit many of these imaging techniques. Although controlled studies with long-term follow-up are necessary to clarify all these controversies, our proposal for tracking the Colombian population is to perform a third-level prenatal ultrasound in all pregnant women to search for malformations such as hydronephrosis. In addition, a renal ultrasound should be performed after the fifth day of life in newborns prenatally diagnosed with hydronephrosis, and even if this test is normal, a second ultrasound should be performed between the fourth and sixth weeks of life. Nonetheless, in the patient with persistent hydronephrosis, ultrasound monitoring must be ensured until resolution, and if there is no improvement or progression, a VCUG should be performed. If the VCUG is normal and hydronephrosis persists, an obstructive process must be excluded by diuretic renography or excretory urography.
REFERENCES
1. Galiano R, Spasari E. Postnatal management of newborn with antenatal detected urinary tract abnormalities. J Matern Fetal Neonatal Med. 2011 Oct;24 Suppl 1:107–10. [ Links ]
2. Piepsz A. Antenatally detected hydronephrosis. Semin Nucl Med. 2007 Jul;37(4):249–60. [ Links ]
3. Hodges SJ. Pre- and postnatal management of hydronephrosis. Scientific World Journal. 2010 Jan;10:2350–1. [ Links ]
4. Yiee JH, Tasian GE, Copp HL. Management trends in prenatally detected hydronephrosis: national survey of pediatrician practice patterns and antibiotic use. Urology. 2011 Oct;78(4):895–901. [ Links ]
5. Lee RS, Cendron M, Kinnamon DD, Nguyen HT. Antenatal hydronephrosis as a predictor of postnatal outcome: a meta-analysis. Pediatrics. 2006 Aug;118(2):586–93. [ Links ]
6. Riccabona M. Assessment and management of newborn hydronephrosis. World J Urol. 2004 Jun;22(2):73–8. [ Links ]
7. Mallik M, Watson AR. Antenatally detected urinary tract abnormalities: more detection but less action. Pediatr Nephrol. 2008 Jun;23(6):897–904. [ Links ]
8. Belarmino JM, Kogan BA. Management of neonatal hydronephrosis. Early Hum Dev. 2006 Jan;82(1):9–14. [ Links ]
9. Estrada CR. Prenatal hydronephrosis: early evaluation. Curr Opin Urol. 2008 Jul;18(4):401–3. [ Links ]
10. Hwang HH, Cho MH, Ko CW. The necessity of voiding cystourethrography in children with prenatally diagnosed hydronephrosis. J Int Med Res. 2011 Jan;39(2):603–8. [ Links ]
11. Yamaçake KGR, Nguyen HT. Current management of antenatal hydronephrosis. Pediatr Nephrol. 2013 Mar;28(2):237–43. [ Links ]
12. Gökaslan F, Yalçinkaya F, Fitöz S, Özçakar ZB. Evaluation and outcome of antenatal hydronephrosis: a prospective study. Ren Fail. 2012 Jan;34(6):718–21. [ Links ]
13. Thom RP, Rosenblum ND. A translational approach to congenital non-obstructive hydronephrosis. Pediatr Nephrol. 2013 Sep;28(9):1757–61. [ Links ]
14. Mesrobian H-GO, Mirza SP. Hydronephrosis: a view from the inside. Pediatr Clin North Am. 2012 Aug;59(4):839–51. [ Links ]
15. Yiee J, Wilcox D. Management of fetal hydronephrosis. Pediatr Nephrol. 2008 Mar;23(3):347–53. [ Links ]
16. Riccabona M, Simbrunner J, Ring E, Ruppert-Kohlmayr A, Ebner F, Fotter R. Feasibility of MR urography in neonates and infants with anomalies of the upper urinary tract. Eur Radiol. 2002 Jun;12(6):1442–50. [ Links ]
17. Hadjidekov G, Hadjidekova S, Tonchev Z, Bakalova R, Aoki I. Assessing renal function in children with hydronephrosis - additional feature of MR urography. Radiol Oncol. 2011 Dec;45(4):248–58. [ Links ]
18. Becker A, Baum M. Obstructive uropathy. Early Hum Dev. 2006 Jan;82(1):15–22. [ Links ]
19. Piedrahita Echeverry V, Prada Meza M, Vanegas Ruiz J, Vélez Echeverry C, Serna Higuita L, Serrano Gayubo A, et al. Causas de enfermedad renal crónica en niños atendidos en el Servicio de Nefrología Pediátrica del Hospital Universitario San Vicente de Paúl, de Medellín, Colombia, entre 1960 y 2010. Iatreia. 2011;24(4):347–52. [ Links ]
20. Gokce I, Biyikli N, Tugtepe H, Tarcan T, Alpay H. Clinical spectrum of antenatally detected urinary tract abnormalities with respect to hydronephrosis at postnatal ultrasound scan. Pediatr Surg Int. 2012 May;28(5):543–52. [ Links ]
21. Rao PK, Palmer JS. Prenatal and postnatal management of hydronephrosis. ScientificWorldJournal. 2009 Jan;9:606–14. [ Links ]
22. Piepsz A. Antenatal detection of pelviureteric junction stenosis: main controversies. Semin Nucl Med. 2011 Jan;41(1):11–9. [ Links ]
23. Asl AS, Maleknejad S. Clinical outcome and followup of prenatal hydronephrosis. Saudi J Kidney Dis Transpl. 2012 May;23(3):526–31. [ Links ]