INTRINSIC FACTORS ASSOCIATED WITH AGING
Cellular senescence (also known as natural aging) is caused by intrinsic factors and is determined by both, physiological factors and genetic predisposition1. Different signs of aging occur as cellular proliferation and hormone levels decrease, or as a result of diverse factors: telomere shortening and accumulation of dysplastic keratinocytes; degradation of the extracellular matrix; mutations in nuclear and mitochondrial genes, and multiple lipid or amino acid metabolic aberrations2)(3)(4. Such disturbances cause functional and physical changes due to abnormal water distribution or a lack of hygroscopic substances, resulting in a dry appearance of the skin. In addition, there is an increase in skin surface pH and a continuous production of reactive oxygen species (ROS) in the mitochondria that results from an oxidative cell metabolism and a decrease in antioxidant activity5)( 6). Also in vivo studies have shown that the expression of connective tissue growth factor (CTGF) and a reduced signaling of the transforming growth factor (TGF)-beta/ Smad are probably responsible for type I procollagen expression loss in intrinsically aged skin7. Finally, alterations in the cutaneous immunological barrier are also part of the aging process that frequently relate to or cause certain skin disorders8.
EXTRINSIC FACTORS ASSOCIATED WITH AGING
Environmental factors such as smoking, automobile contaminants, alcohol consumption, dietary habits, industrial waste and lifestyle options can contribute to aging6)(9)(10. Ultraviolet radiation (UVR) from the sun or artificial sources has a deleterious effect on skin functions and keratinocyte survival, a process known as photoaging10)(11. Additionally, according to recent data, visible light and infrared radiation also can lead to skin damage.
SUNLIGHT RADIATION SPECTRUM
Sunlight is composed of three different types of UV radiation: UVC (100-290 nm) which is largely blocked by the ozone layer and therefore has little impact on the skin; UVB (290-320 nm) which penetrates mainly into the epidermis (stratum corneum) and is responsible for the erythema associated with a sunburn, and UVA (320-400 nm), which penetrates into the dermis, and has the main role in chronic skin damage. These types of radiation represent about 10 % of the total solar radiation reaching the atmosphere. On the other hand, visible light (400-780 nm) and infrared radiation (780-1400 nm), which represent around 40 % and 50 % of the total radiation emanating from the sun, respectively, are also important factors in photoaging because they can exert both acute and chronic deleterious cutaneous effects14)(15.
MOLECULAR MECHANISMS OF SKIN DAMAGE INDUCED BY SUNLIGHT
Ultraviolet skin exposure initiates a flurry of molecular and cellular responses that end up with several dynamic internal disorders16. DNA is one of the major UV-absorbing molecules chemically altered by such exposure which also induces accumulative mutations17. UVB acts directly inducing the formation of cyclobutane pyrimidine dimers and photoproducts4)(5)(6. Such damage interferes with replication, causes transcription disruption, and induces mutations mainly within the tumor suppressor gene p53, altering the control of the cellular cycle; such alteration increases the risk of keratinocyte and melanocyte transformation that eventually manifests as skin tumors17)(18)(19. On the other hand, UVAradiation acts indirectly by stimulating ROS and/ or free radicals production. Reactive oxygen species accumulation is perhaps one of the most important cellular events after solar exposure as it induces the following cellular alterations:
Nuclear and mitochondrial DNA mutations as a result of a modification of guanine (8-hydrox- 2′-deoxy guanine), single-stranded breaks, and oxidized pyrimidine bases20)(21.
Membrane protein carbonilation and lipid peroxidation22.
Apoptosis of epidermal keratinocytes (sunburn cells)23.
Release of keratinocyte proinflammatory cytokines (mainly IL-1, IL-6, TNF-α) and dermal fibroblasts growth factor receptors such as the epidermal growth factor receptor (EGFR), tumor necrosis factor α (TNF-α), platelet activation factor (PAF) prostaglandins, and insulin. Another transcription factor important in photoaging is the nuclear factor kappa β (NF-kβ) which is also activated by UV radiation and has an effect on matrix proteins because it stimulates the transcription of inflammatory cytokines which attract neutrophils that also express matrix metalloproteinases (metalloproteinase-8) with concomitant extracellular matrix proteins degradation24)(25)(26)(27)(28)(29. Transcription of metalloproteinases (MMPs) is dependent on transcription factor AP1, and similarly, AP1 depends on the activation of MAPK signaling pathway by ROS in keratinocytes30. Also, dermal matrix metalloproteinases upregulation stimulates the production of collagenase, gelatinase and stromelysin-1 in both fibroblasts and keratinocytes, resulting in a deterioration of both collagen and elastin, as well as of other components of the dermal extracellular matrix. While the expression of metalloproteinases is activated, inhibitors of these enzymes are also reduced30)(31)(32.
A decrease in the expression of TGF-β, which brings down an altered collagen production and enhances elastin production thereby producing changes in skin structure which clinically manifest by deep wrinkles, coarse textures, telangiectasia and pigmentation33)(34.
Local and systemic immunosuppression by activation of immunosuppressive molecules such as IL4, IL10, IL-1β, TNF-α, and PGE2, or by alteration in cellular morphology, function or quantity of Langerhans cells and T lymphocytes27)(35)(36)(37, are other consequences of ROS production after UV-radiation exposure. Antigen presentation in UV exposed skin is also impaired mainly due to suppression of important molecules expression such as MHC class II, lymphocyte function associated antigen-3 (LFA-3), ICAM-1, ICAM-3, B7, CD1a and CD4017)(38)(39. UV skin radiation also activates the local glucosteroidogenesis process in skin and this in turn leads to the attenuation of local cutaneous immunity40.
All mentioned crucial alterations of the immune system favor a state of cellular malignant transformation that manifests as cutaneous neoplasias17. In addition, there are several skin diseases that can be aggravated (e.g idiopathic photodermatoses, phototoxic contact dermatitis, photoallergic contact dermatitis, photosensitivity caused by medications, etc.); influenced (melasma, vitiligo, lupus erythematosus, seborrheic dermatitis, psoriasis, etc.); caused or triggered by resultant UVRinduced immunosuppression (e.g infections such as herpes simplex, and viral exanthemas)15.
SKIN CHANGES INDUCED BY SMOKING
It has been described that heavy smoking is strongly associated with poor wound healing, certain squamous cell carcinomas, oral cancer, psoriasis exacerbations, and premature skin aging41)(42)(43. Such skin damage induced by tobacco has been related to oxidative stress, impaired collagen biosynthesis, and activation of matrix metalloproteinases. In addition, tobacco smoking air pollutants activate the aryl hydrocarbon receptor (AhR) pathway, a liganddependant transcription factor that mediates toxicity induced by several environmental contaminants, which may play a role in already known premature skin-aging effects44. Figure 1 summarizes the main events induced by UVA, UVB and smoking.
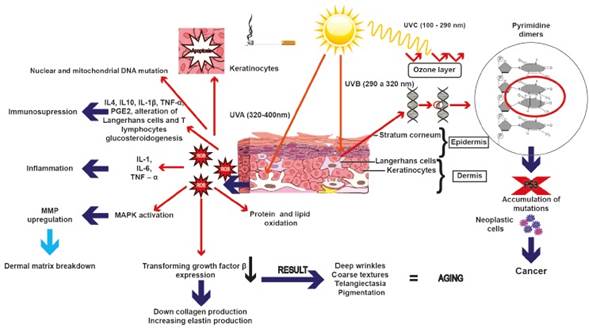
Figure 1 Ultraviolet radiation and cigarette smoking affect the stability of DNA, causing alteration in molecule structure due to the formation of mutations that accumulate and promote cellular malignant transformation. Also, there may be an accumulation of ROS due to p53 inability to remove them, which in turn can lead to inflammation by activating the expression of certain interleukins as well as immunosuppression or the overexpression of metalloproteinases
MECHANISMS OF SKIN PROTECTION
Human beings have been constantly exposed to sunlight radiation. As the outermost organ, skin acts as the main protective barrier against UV-radiation and other extrinsic agents45)(46. The cutaneous barrier is composed of two primary layers: epidermis and dermis. Each one exhibits unique structural and physiological functions that have important roles against extrinsic factors. Recent advances in the understanding of photodamage mechanisms have enhanced strategy development to prevent alteration of the structure and function of the skin. The first step in order to prevent acute and chronic damage development by UVradiation is photoprotection, which can be understood as a group of measures directed to reduce exposure; this can be accomplished with a set of strategies that involve photoeducation, topical photoprotection (sunscreens), oral photoprotection and photoprotection by devices (clothes, hats, etc.)15.
Regarding oral and topical photoprotection, exogenous nutrients such as α-tocopherol, oral and topical retinoids such as vitamin A and β-carotene (a precursor of vitamin A), lycopene, and lutein all contribute to the formation of a UV-radiation barrier and some of them act as antioxidants47. Indeed, it has been shown that retinoids protect skin from UVradiation because they have the ability to increase the proliferation of epidermal keratinocytes and dermal fibroblasts48, and/or inhibit the expression of matrix metalloproteinases (MMPs), matrix-degrading enzymes, leading to an increase in overall protein and extracellular matrix content30)(32.
On the other hand, vitamins E and D also protect skin from ROS damage. In fact, it has been described that vitamin E inhibits MMP-1 expression, thus avoiding collagen breakdown, and acts as an anti-inflammatory agent, whereas vitamin D has been shown to stimulate innate immunity49)(50)(51.
Previous work has suggested a role of mineral elements such as zinc, copper, and selenium in preventing skin aging. These elements are cofactors of important enzymes that act in protein synthesis of the extracellular matrix, but also have other functions such as radiation absorbance (e.g. zinc)52. Copper is also crucial in this process because it acts as an antioxidant and stimulates collagen and melanin synthesis53. Lastly, selenium has been described to protect skin against oxidative stress54.
Intrinsic skin factors such as the local steroidogenic system also have an important role in cutaneous protection against harmful effects of UV-radiation as it is capable of synthesizing androgens, estradiols, gluco- and mineralo-corticoids from precursors, all of which play anti-cancer roles by getting converted into secosteroids under the influence of UV-radiation55. Additionally, chromophores such as urocanic acid, which is formed from L-histidine in the stratum corneum, is a potent endogenous UV-absorbant56. Nevertheless, melanin, an UV-absorbing pigment that is able to dissipate UV radiation as harmless heat, is probably the most important skin constituent that exerts a defensive function against UV radiation damage. Such pigment is produced by melanocytes and transferred to keratinocytes via long dendritic processes17)(57 and acts as a UV filter that inhibits dimers and 6,4 photoproducts formation or as a ROS scavenger58)(59. In addition, melanin is responsible for skin color which has been suggested is the result of an adaptive response to UVR as skin darkness observed in certain communities relates with the levels of UVR which they are exposed to. It has been suggested that such color changes take place in order to decrease vitamin D synthesis requirements in the skin. Such skin adaptation also seems to prevent UV damage of sweat glands which would have a major impact on thermoregulation, especially in warm areas60)(61. Also, melanin levels have been shown to be inversely correlated with the amount of skin DNA damage induced by UV; therefore, it seems that white Caucasians low pigmentation capacity is responsible for a failure in intrinsic photoprotection mechanisms62)(63.
Another important component in epidermal protection is melatonin, a hormone synthesized by the pineal gland, which is capable of protecting epidermal keratinocytes from oxidative stress by suppressing ROS generation and increasing a reduced glutathione content64)(65. In addition, the skin has natural endogenous enzymatic antioxidants (e.g. superoxide dismutase or catalase) and non-enzymatic antioxidants (e.g. coenzyme Q10, glutathione or vitamin E), which also provide protection against ROS66.
Human skin is also protected from UV radiation damage by the activation of complex molecular repair mechanisms that correct DNA-injuries in order to maintain genomic integrity67. The most important mechanism in this process is nucleotide excision DNA repair (NER) which is in charge of cyclobutane pyrimidine dimers and (6-4) photoproducts removal. Indeed, malfunction of this repair system is related to skin disorders such as xeroderma pigmentosum and photoaging63)(68. Another repairing mechanism is base excision repair (BER) which removes oxidative modifications on a single base in DNA69.
Another mechanism of reparation by NER and BER is via p53 activation by inducing arrest at G2/M checkpoint70. If serious damage occurs, p53-dependent apoptosis takes place in order to get rid of damaged DNA and to prevent cancer occurrence17)(71)(72. Also, it has been demonstrated that upregulation of IL-12 can counteract UVB-induced immunosuppression73.
FUTURE APPROACHES FOR SKIN PROTECTION WITH NATURAL PRODUCTS
In modern society, there is a great increase in the search for eternal youth74. For this reason, new therapeutic options are being explored and many natural active ingredients seem to offer alternatives for skin protection75. Skin protection obtained with some natural products is given by their antioxidant effect, melanin production or activation of transcription factors76)(77)(78)(79. Indeed, the plant Rosmarinus officinalis is a source of rosmarinic acid, which acts as a free radical scavenger and stimulates melanin production76)(77. Recently, it has been described that topical application of forskolin (a product derived from the plant Plectranthus barbatus (Coleus forskolii) up-regulates melanin production via a direct activation of adenylate cyclase that induces the production of cAMP, which results in melanin deposition. This event is melanocortin-1 receptor (MC1R) dependent which is a protein located in the extracellular membranes of epidermal melanocytes80. Also, paeoniflorin (another natural product extracted from the plant Paeonia lactiflora), has been shown to have the ability to repair DNA and probably to reduce facial wrinkles in human skin81.
In various molecules of natural origin (either in the form of plant extracts, fruits and/or tubers), a role has been found in the activation of nuclear factor erythroid 2-like 2 (Nrf2) (a master transcription factor which regulates a battery of genes involved in defense mechanisms against ROS damage). Therefore, compounds that act as activation enhancers of this factor might be useful in prevention of skin damage and/or malignant transformation under various stress conditions78.
In the last decade, some herbal sunscreens have replaced sunscreens containing chemical UV-filters due to the side effects associated with the latter. Many herbal sunscreens are currently available in the market in the forms of creams, lotions, and/or gels formulations which have included a labeled sun protector factor (SPF)82. In addition, plants characterized for protecting themselves from intense heat and UV radiation could be promising in future formulations83. Other innovative options for photoprotection are DNA-oligonucleotides, which enhance DNA-repair and stimulate melanogenesis84.
In addition, antiaging properties have not only been found in plants. Previous studies have described regenerative properties and an antioxidant activity in mollusk Cryptomphalus aspersa85. Also, it has been recently shown that C. aspersa eggs extract promotes migration and prevents cutaneous aging in keratinocytes and dermal fibroblasts in vitro86.
Lastly, different peptides represent an emerging field in cosmeceuticals for photoaging because they have cosmetically interesting activities such as collagen synthesis and chemotaxis stimulation and antistinging effects87)(88.
The majority of publications in the field of natural products and essential oils for potential use in cosmetics are from China and India83)(89)(90)(91)(92. In Latin America, the main publications in this area are form Brazil, Argentina, Mexico and Uruguay, whereas Colombia has published very few studies92)(93)(94. In fact, instead of looking for a direct effect in skin photoaging, most Colombian studies have been focused on the antioxidant or photo-protective activity of some products. One Colombian report has showed the in vitro antioxidant activity of two types of leaves: The Colombian Magnoliaceae and Talauma hernandezii95. In such study, the activity of these magnoliaceae was determined using 2,2′-azino-bis(3- ethylbenzothiazoline-6-sulfonic acid) (ABTS·+) and the stable free radical 2-2-diphenyl-1-picrylhydrazyl (DPPH·), and antioxidant properties obtained were similar to those reported from α-tocopherol, tertbutylated hydroxyanisole (BHA), and ascorbic acid95.
By the use of the free radical α-α-diphenyl- βpicrylhydrazyl (DPPH· ) and the preformed radical monocation 2,2′-azino-bis(3-ethylbenzothiazoline-6- sulphonic acid) (ABTS·+), another Colombian study has proven to have an antioxidant activity comparable to that of α-tocopherol, BHA, ascorbic acid and Troloxin. Some of the products under investigation in this study had antioxidant capabilities in essential oils: from anise (Pimpinella anisum L.), fennel (Foeniculum vulgare Mill.), coriander (Coriandrum sativum L.), rosemary (Rosmarinus officinalis L.), oregano (Origanum vulgare L.), ylang-ylang (Cananga odorata Hook.), verbena (Lippia alba Mill.) and salvia negra (Lepechinia schiedeana (Schlecht) Vatke, syn. Stachys schiedeana Schlecht)96. Another Colombian study has checked the antioxidant and protective abilities of Lobariella pallida and Stereocaulon strictum var. compressum extracts to prevent cell and DNA damage by oxidative stress97. Another study from this same research group has evaluated the antioxidant and photo-protective efficacy of the extract and isolated compounds from the lichen Usnea rocellina Motyka, by evaluating the antioxidant activity through the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and ferric reducing power98.
In conclusion, skin aging is a natural process in which different cellular and molecular mechanisms, induced by intrinsic and extrinsic factors, are involved. Currently, increasing attempts are being made to delay skin aging via the ingestion or application of natural products or by formulating new natural sunscreens with higher radiation protective capability. In this respect, plant biodiversity in Latin American countries, and particularly in Colombia, are important sources of potential candidates for topical use. However, anti-aging products innovation requires knowledge of the mechanisms that trigger cell damage due to intrinsic or to extrinsic deleterious factors. More importantly, as the scientific support for the use of the aforementioned products is based on in vitro data, there is a need for a formal evaluation of such products in high quality and well-designed preclinical and phase-1 and -2 clinical trials in order to assess the best dosage and also to determine their real safety and efficacy.
ACKNOWLEDGEMENTS
We are grateful to María Constanza Patiño and to Eduardo García for their technical assistance in language review of the article. Sindy Flórez was sponsored through a grant from the University of Antioquia “Jóvenes Investigadores” program, years 2014-2015. We also thank María Isabel González Gaviria (Facultad de Arquitectura, Universidad Pontificia Bolivariana, Medellín, Colombia), for her assistance in the design of figures.














