Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista de la Universidad Industrial de Santander. Salud
versão impressa ISSN 0121-0807
Rev. Univ. Ind. Santander. Salud vol.45 no.1 Bucaramanga jan./abr. 2013
New agents with potential leishmanicidal
activity identified by virtual
screening of chemical databases
New agents with potential leishmanicidal activity
Juan Rebollo, BS1, Jesús Olivero-Verbel, PhD2, Niradiz Reyes, PhD1
1. Genetics and Molecular Biology Group. School of Medicine. University of Cartagena, Cartagena, Colombia.
2. Environmental and Computational Chemistry Group. School of Pharmaceutical Sciences, University of Cartagena, Cartagena, Colombia.
Correspondence: Niradiz Reyes, PhD Address: School of Medicine. Department of Basic Sciencies.Campus of Zaragocilla. University of Cartagena.Cartagena - Colombia Telephone number: (+57) 5 6698178 ext 140 Fax number: (+57) 5 6698178. E-mail: nreyesr@unicartagena.edu.co
Recibido: 07 de octubre de 2012 Aprobado: 25 de febrero de 2013
ABSTRACT
Introduction and Objectives: Leishmaniosis, a disease caused by a protozoan parasite, remains a serious public health problem threatening about 350 million people around the world, of which 12 million are believed to be currently infected (WHO 2010). To date, there are no vaccines against the species of parasites and the treatment is based only on chemotherapy with toxic-, expensive- and inefficient- drugs. There is an urgent need for better drugs against Leishmania, the etiological agent of the disease. The main anti-leishmanial drug used in Colombia is meglumineantimoniate [chemical name according to the International Union of Pure and Applied Chemistry (IUPAC): Hydroxy-dioxostiborane; (2R,3R,4R,5S)- 6-methylaminohexane-1,2,3,4,5-pentol, (C7H17NO5)], which is not efficient in the treatment of infections caused by Leishmania braziliensis, the most prevalent specie in the Caribbean coast of Colombia. Methods: We performed an in silico virtual screening of several datasets including ChemBridge and Pubchem. We virtually screened a total of 28.755 compounds against a 3D model of 6-phosphoglucono -lactonase (6-PGL) from Leishmania braziliensis to identify novel inhibitors.Molecular docking of databases was performed using the software Sybyl 8.0 and AutoDockVina. Results: The initial virtual screening using a structure-based method identified 10 compounds, which were later tested with AutodockVina and classified according to their docking scores. Conclusions: These novel and potential inhibitors constitute new drug candidates that must be biologically tested to define their value as an alternative chemotherapeutic agent in the treatment of these protozoan infections. Salud UIS 2013; 45 (1): 33-40
Keywords: leishmaniosis, virtual screening, therapeutics, molecular docking simulation, drug search.
Evidence level: III
Nuevos agentes con potencial actividad leishmanicida
identificados mediante tamizaje virtual
RESUMEN
Introducción y Objetivos: Leishmaniosis, una enfermedad causada por un parásito protozoario, representa un serio problema de salud pública que amenaza a cerca de 350 millones de personas alrededor del mundo, de los cuales se cree que unos 12 millones se encuentran actualmente infectados (WHO 2010). A la fecha no existen vacunas contra las especies del parásito y el tratamiento está basado solo en la quimioterapia con medicamentos tóxicos, costosos, e ineficientes. Existe una necesidad urgente por mejores medicamentos contra Leishmania, el agente etiológico de la enfermedad. El principal medicamento en Colombia usado contra la leishmaniosis es el antimoniato de meglumine [nombre químico según los parámetrosde la International Union of Pure and Applied Chemistry (IUPAC): Hydroxy-dioxostiborane; (2R,3R,4R,5S)-6- methylaminohexane-1,2,3,4,5-pentol, (C7H17NO5)], el cual no es eficiente en el tratamiento de infecciones causadas por Leishmania braziliensis, la especie más prevalente en la costa Caribe de Colombia. Métodos: En este trabajo efectuamos un tamizaje virtual in silico de varias bases de datos incluyendo ChemBridge y Pubchem. Con el objetivo de identificar nuevos inhibidores, un total de 28.755 compuestos fueron tamizados virtualmente contra un modelo 3D de la enzima 6-phosphoglucono -lactonase (6-PGL) de Leishmania braziliensis. El acoplamiento molecular de las bases de datos se efectuó con el programa Sybyl 8.0 y AutoDock Vina. Resultados: mediante tamizaje virtual basado en la estructura se identificaron10 compuestos, los cuales fueron posteriormente evaluados con AutodockVina y clasificados de acuerdo a los puntajes de acoplamiento. Conclusiones: Estos nuevos potenciales inhibidores constituyen candidatos a medicamentos que deben ser evaluados biológicamente para definir su valor como alternativas quimioterapéuticas en el tratamiento de estas infecciones parasíticas. Salud UIS 2013; 45 (1): 33-40
Palabras clave: leishmaniosis, tamizaje virtual, terapéutico, biblioteca virtual, base de datos.
Nivel de evidencia: III
INTRODUCTION
Leishmaniosis is a disease caused by protozoan parasites of the genus Leishmania1. It is considered by the World Health Organization (WHO) as a serious public health problem threatening about 350 million people in 88 countries around the world of which 12 million are believed to be currently infected2. Leishmaniosis has been classified as endemic in 98 countries or territories, including tropical and subtropical regions of Africa, Asia, Europe, North, Central and South America3. In Colombia, the disease is a growing problem, reported by the National Institute of Health with about 15 000 new cases in 20104, and the appearing of new sources of transmission reflect an increase in the spread of Leishmania (L.) strains5.
There are no vaccines against these parasites and the approaches to control the vector are not effective. The main line of defense against the disease is chemotherapy which is not efficient against the different species of the parasite, and patients commonly suffer toxic side effects, including heart, kidney, liver and gastrointestinal failure; worsened by the long time drug administration and the increasing parasite resistance. In addition, Meglumine antimoniate [chemical name according to the International Union of Pure and Applied Chemistry (IUPAC): Hydroxy-dioxostiborane; (2R,3R,4R,5S)-6- methylaminohexane-1,2,3,4,5-pentol, (C7H17NO5)], the first line of treatment currently used in Colombia6, has led to progressive rise in treatment failure7-10.On the other hand, Miltefosine, a drug effective against infections caused by L. panamensis, is not effective against infections produced by L. braziliensis (cure rates below 50%)11, which is the most prevalent specie reported in the Caribbean region of the country12. Thus, there is an urgent need for new drugs against this parasite. Despite this situation, leishmaniosis is still a neglected tropical disease (NTDs)13 prevalent in developing countries and poor populations14. Therefore, implementation of rational and cost effective approaches to treat this health problem is required. In this regard, virtual screening, and in particular receptor-based virtual screening, has emerged as a reliable, inexpensive method for identifying leads 15, and the in silico screening of chemical databases to search for new compounds against Leishmania would allow us to identify specific inhibitors of target molecules of the parasite.
The current study describes a rational scheme in the quest for new Leishmania targets, through the study of its biochemical pathways, and shows the potential of structure based virtual screening to identify novel compounds with inhibitory activity against essential target enzymes from the parasite using computer aided molecular docking of databases16,17.
MATERIAL AND METHODS
Selection of target proteins
To carry out virtual screening using molecular docking, we first selected the most appropriate target protein of the parasite, which met the following selection criteria: the putative target should be involved in a biochemical pathway essential for survival of the parasite cell; it should have low homology to its counterpart in the human host; and it may have a suitable three dimensional model in Protein Data Bank (PDB).
Potential targets were identified through a systematic search of relevant scientific literature on Leishmania metabolism indexed in MEDLINE database and retrieved through PubMed search engine, using key words "Leishmania metabolism", "Leishmania drug targets" and "Leishmania metabolic pathways".
Homology of each selected protein to their human counterpart was determined with the PSI-BLAST program from the European Bioinformatics Institute (EMBL-EBI).
Modeling the three dimensional structure of selected targets
A search for the three dimensional structure of each protein on Protein Data Bank (PDB) was performed. For proteins lacking a defined three-dimensional structure, experimental models were developed with integrated SYBYL8.0 Orchestra, and the servers I-TASSER 18, EsyPred3d 19 and 3D-JigSaw 20.
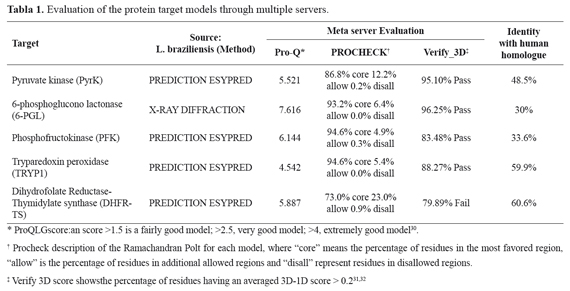
In order to select the best model, the 3D structure of each potential target was assessed by ProQ-Protein Quality Predictor Server, SWISS-MODEL and SAVES, a meta-server for analysis and verification of models.
Selection of compound datasets
Diverse chemical datasets used in the study were obtained from ChemBridge Corporation portfolio and PubChem database, containing 20.000 and 8.755 compounds, respectively. Molecules from PubChem were selected based on their structural relationship with the natural ligands of selected protein targets from Leishmania. SDF files available online and containing two dimensional coordinates of the compounds were downloaded for each collection, and a Sybyl database was built.
Protein and ligand preparation
The protein chosen in our study was the 6-phosphoglucono- lactonase (6-PGL) from L. braziliensis. The three dimensional structure of 6-PGL from Leishmania braziliensis (PDB ID: 3CH7) resolved by X-ray diffraction at 2.29 Å was downloaded from the Protein Data Bank. The protein was edited, converted to mol2 format and processed for docking procedure using the Sybyl docking interface. Water molecules were removed before docking simulation, the protomol was built through the automatic mode and confirmed by alignment with the already determined active site of 6-PGL from Trypanosoma brucei (PDB ID: 3E7F). For the Vina based docking, 6-PGL was processed with the Autodock tools (ADT) software, water moleculeswere removed, polar hydrogen atoms added and Kollman charges assigned.
The ligand datasets in SDF format were converted to a Sybyl database in mol2 format and each compound was minimized by Sybyl energy minimizer using the Tripos force field and Gasteiger-Hückel charges. The maximum number of interactions was set to 100 and the minimization was terminated by the Powell method, when the energy gradient of 0.05 kcal/mol/Å was reached.
The analysis by AutodockVina required the conversion of the ligands and protein to the PDBQT format, which is the input format for AutoDockVina calculations.
Structure Based Virtual screening
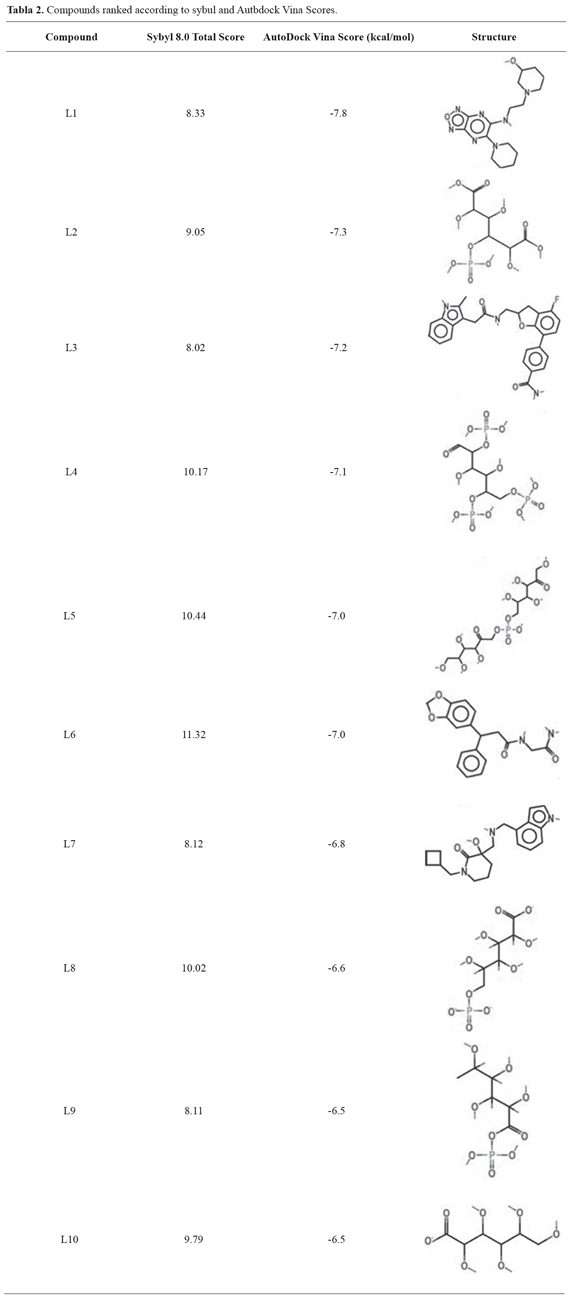
Virtual screening of 28.755 compounds against the 3D structure of 6-PGL was performed with the Surflex-Dock application coupled in Sybyl 8.0. The ligands were later classified according to the total score and the best docked compounds (Total Score > 8) were selected for testing using the AutoDockVina software. The selected ligands were docked to a rigid receptor limited by the coordinates of the grid, which was defined using the ADT application and the exhaustiveness of the docking was set to nine. Each compound was docked individually. The best dockings were manually inspected and their interactions with the receptor were described
RESULTS AND DISCUSSION
Selection of target proteins A rational approach to search and design new antileishmanial compounds must select a suitable target protein, which should be essential for the parasite survival and as different as possible from its human counterpart. A systematic review of the literature searching for potential target molecules involved in Leishmania metabolism identified five enzymes as potential targets for drugs. These biochemical targets are involved in glycolysis (phosphofructokinase and pyruvate kinase), the pentose phosphate pathway (6-phosphogluconolactonase), nucleotide metabolism (dihydrofolatereductase) and antioxidants (tryparedoxin peroxidase), all of them considered essential for the survival of Leishmania21,22..
Enzymes involved in glycolysis appear to be appropriate therapeutic targets because they are essential for the amastigote form of the parasite. In addition, there is a huge evolutionary distance between Leishmania and Homo sapiens that make the parasite proteins, involved in this pathway, structurally different from their mammalian equivalents23. The phosphofructokinase (PFK), an enzyme present in the amastigote form of Leishmania, participates in this biochemical pathway, catalyzing the phosphorylation of fructose-6-phosphate (F6P) to produce fructose 1,6-bisphosphate24 and is structurally different from the human PFK, a feature useful for the designing of specific inhibitors ofits biological function. On the other hand, pyruvate kinase (PyK) catalyzes the conversion of phosphoenol pyruvate (PEP) to pyruvate to produce ATP. This enzyme is allosterically regulated by fructose 2,6-bisphosphate, which induces the conversion to the active state of the protein22. Studies that compared the PyK crystal structure of the parasite withthe PyKof other organisms have identified important differences between these enzymes at the level of its effector site revealing unique regulatory properties for the parasite enzyme, which could be a potential target for drugs25,26.
The trypanothione metabolism is another biochemical pathway with potential targets; the enzyme tryparedoxin peroxidase (TryP) is a key molecule in the defense mechanism against oxidative stress27. This enzyme is responsible for the removal of reactive oxygen intermediates such as superoxide anion (O2-), hydrogen peroxide (H2O2), peroxynitrite (ONOO-) and hydroxyl radical (OH-) produced during the process of cellular respiration. Furthermore, studies with mutants of L. donovani and L. major lacking a functional trypanothione reductase have established that this enzyme is essential for parasite survival28. Another important enzyme that protects Leishmania from toxic radicals is the 6-Phosphogluconolactonase from the pentose phosphate pathway. It converts 6-phosphogluconolactone to 6-phosphogluconate maintaining a source of NADPH, which serves to protect against oxidative stress and generates carbohydrate intermediates used in nucleotide and other biosynthetic pathways. Furthermore, the loss of this enzyme has been shown to be toxic for the parasite, making it a suitable target for chemotherapy29.
Modeling the three dimensional structure of selected targets
The only enzyme reported in the PDB with a threedimensional structure defined by X-ray diffraction was the 6-Phosphogluconolactonase. For the other enzymes, it was necessary to build their threedimensional models from the amino acid sequence of each protein through different modeling programs (I-TASSER, 3D-JigSaw and Orchestra, included in Sybyl 8.0). After evaluation of each model by the meta server SAVS (Structural Analysis and Verification Server), the most suitable model was selected for docking analysis. Considering the quality of the threedimensional model retrieved from the PDB and the three basic characteristics that a target must satisfy (Table 1.), the protein and model chosen was the 6-PGL from L. braziliensis.
Virtual Screening
Structure-based virtual screening was carry out to predict the binding affinity of 28.755 molecules from a diverse chemical library, in order to identify novel drug-like inhibitors of 6-PGL from Leishmania. This method involved computational docking of ligands into an active site of a receptor using complex algorithms, followed by scoring and ranking of these compounds to identify potential leads. Compared to ligand- or pharmacophore- based method, the receptor-based method of screening is a more flexible approach that allows the identiï¬cation of structurally novel ligands that may have similar interactions to those of known ligands, or may have different interactions with other parts of the binding site33.
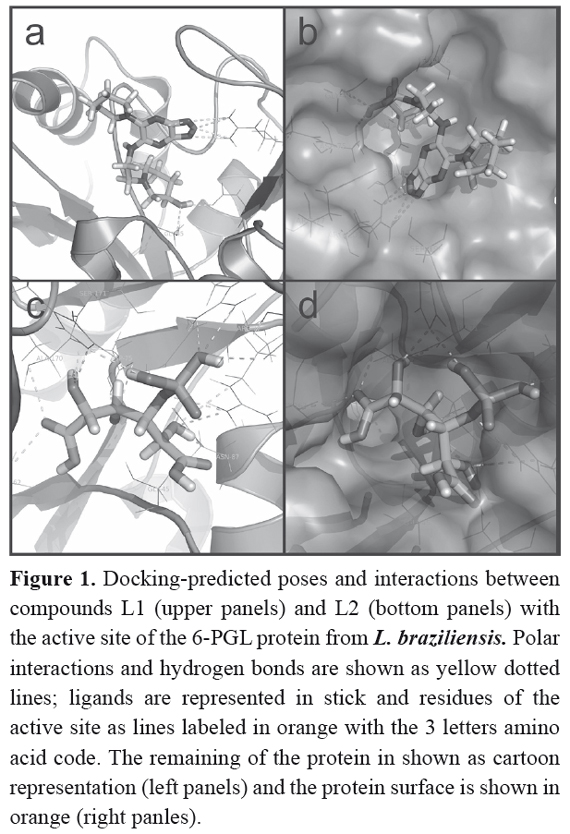
The first screening, performed with Sybyl 8.0 program, provided a set of 11 candidate molecules, which bound to the receptor with the higher affinities (Total Score > 8) (Table 2). These ligands were later docked to the 6-PGL through the AutodockVina program to validate the binding affinities and to sort them according to their docking scores (Table 2).Compounds listed in this table could be considered as potential leads on the light of virtual docking, but should be tested in vitro or in vivo assays to confirm their real potential as anti-leishmanial drugs. Figure 1 shows the molecular docking of compound L1 with the active site of 6-PGL from Leishmania; the ligand is maintained in the active site by a set of intermolecular forces that include polar interactions and hydrogen bonds.
CONCLUSIONS
Structure-based virtual screening allowed us to identify a set of potential anti-leishmanial compounds through a computer-aided drug discovery approach;we screened in silico a diverse and large library of 28755 compounds against 6-PGL protein, a vital enzyme for Leishmania survival. Ligands bounded with higher affinity to the receptor protein were subjected to docking validation and ranked through the AutodockVina docking score function.
We consider that compounds identified represent candidates for testing as potential therapeutic agents against leishmaniosis. However, the real leishmanicidal activity of these compounds should be biologically evaluated before they can be proposed as anti-leishmanial drugs. Nevertheless, by this computer-aided screening we have identified from a large chemical database, the leads with the higher likelihood of binding to the target protein to elicit an inhibitory activity, which would reduce the cost of biological testing.
ETHICAL CONSIDERATIONS
The authors declare that this manuscript was developed within the current regulations
CONFLICTS OF INTEREST
The authors declare that no conflict of interest exists in the development of this research
ACKNOWLEDGEMENTS
The authors want to thank Carlos Ortega for his valuable collaboration. This work was supported by Universidad de Cartagena Intramural Research Grant # Res 3735 -2009.
REFERENCES
1. Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7(9):581-96. [ Links ]
2. WHO. Control of the leishmaniases. Switzerland; 2010. Contract. [ Links ]
3. Sinha PK, Pandey K, Bhattacharya SK. Diagnosis &, management of leishmania/HIV co-infection. Indian J Med Res. 2005;121(4):407-14. [ Links ]
4. INS. SISTEMA DE VIGILANCIA EN SALUD PÚBLICA - SIVIGILA: Semana epidemiológica No 52. Bogota, Colombia: Instituto Nacional de salud (INS), 2010. Contract. [ Links ]
5. Rodriguez-Barraquer I, Gongora R, Prager M, Pacheco R, Montero LM, Navas A, et al. Etiologic agent of an epidemic of cutaneous leishmaniasis in Tolima, Colombia. Am J Trop Med Hyg. 2008;78(2):276-82. [ Links ]
6. Palacio D, Urquijo L, Nogueira A, Monteiro T. Guía de Atención Integral de Leishmaniasis: Ministerio de la Protección Social, 2010. [ Links ]
7. Faraut-Gambarelli F, Piarroux R, Deniau M, Giusiano B, Marty P, Michel G, et al. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41(4):827-30. [ Links ]
8. Lira R, Sundar S, Makharia A, Kenney R, Gam A, Saraiva E, et al. Evidence that the high incidence of treatment failures in Indian kalaazar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis. 1999;180(2):564-7. [ Links ]
9. Sundar S. Drug resistance in Indian visceral leishmaniasis. Trop Med Int Health. 2001;6(11):849-54. [ Links ]
10. Haldar A, Sen P, Roy S. Use of antimony in the treatment of leishmaniasis: current status and future directions. Molecular biology international. 2011;571242. [ Links ]
11. Soto J, Arana B, Toledo J, Rizzo N, Vega J, Diaz A, et al. Miltefosine for new world cutaneous leishmaniasis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;38(9):1266-338. [ Links ]
12. Martinez LP, Rebollo JA, Luna AL, Cochero S, Bejarano EE. Molecular identification of the parasites causing cutaneous leishmaniasis on the Caribbean coast of Colombia. Parasitol Res. 2010;106(3):647-52. [ Links ]
13. Feasey N, Wansbrough-Jones M, Mabey DC, Solomon AW. Neglected tropical diseases. Br Med Bull. 2010;93:179-200. [ Links ]
14. Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol. 2006;22(12):552-7. [ Links ]
15. Lyne PD. Structure-based virtual screening: an overview. Drug Discov Today. 2002;7(20):1047-55. [ Links ]
16. Tripos. SYBYL. In: International T, editor. 8.0 ed. South Hanley Rd., St. Louis, Missouri, 63144, USA: Tripos International; 1699. [ Links ]
17. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2009;31(2):455-61. [ Links ]
18. Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5(4):725-38. [ Links ]
19. Lambert C, Leonard N, De Bolle X, Depiereux E. ESyPred3D: Prediction of proteins 3D structures. Bioinformatics. 2002;18(9):1250-6. [ Links ]
20. Bates PA, Kelley LA, MacCallum RM, Sternberg MJ. Enhancement of protein modeling by human intervention in applying the automatic programs 3D-JIGSAW and 3D-PSSM. Proteins. 2001;Suppl 5:39-46. [ Links ]
21. Fairlamb AH. Metabolic pathway analysis in trypanosomes and malaria parasites. Philos Trans R Soc Lond B Biol Sci. 2002;357(1417):101-7. [ Links ]
22. Nowicki MW, Tulloch LB, Worralll L, McNae IW, Hannaert V, Michels PA, et al. Design, synthesis and trypanocidal activity of lead compounds based on inhibitors of parasite glycolysis. Bioorg Med Chem. 2008;16(9):5050-61. [ Links ]
23. Shukla AK, Singh BK, Patra S, Dubey VK. Rational approaches for drug designing against leishmaniasis. Appl Biochem Biotechnol. 2010;160(8):2208-18. [ Links ]
24. Haanstra JR, van Tuijl A, Kessler P, Reijnders W, Michels PA, Westerhoff HV, et al. Compartmentation prevents a lethal turbo-explosion of glycolysis in trypanosomes. Proc Natl Acad Sci U S A. 2008;105(46):17718-23. [ Links ]
25. Fothergill-Gillmore LA, Rigden DJ, Michels PA, Phillips SE. Leishmania pyruvate kinase: the crystal structure reveals the structural basis of its unique regulatory properties. Biochem Soc Trans. 2000;28(2):186-90. [ Links ]
26. Tulloch LB, Morgan HP, Hannaert V, Michels PA, Fothergill-Gilmore LA, Walkinshaw MD. Sulphate removal induces a major conformational change in Leishmania mexicana pyruvate kinase in the crystalline state. J Mol Biol. 2008;383(3):615-26. [ Links ]
27. Diechtierow M, Krauth-Siegel RL. A tryparedoxindependent peroxidase protects African trypanosomes from membrane damage. Free Radic Biol Med. 2011;51(4):856-68. [ Links ]
28. Dumas C, Ouellette M, Tovar J, Cunningham ML, Fairlamb AH, Tamar S, et al. Disruption of the trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO J. 1997;16(10):2590-8. [ Links ]
29. Barrett MP. The pentose phosphate pathway and parasitic protozoa. Parasitol Today. 1997;13(1):11-6. [ Links ]
30. Cristobal S, Zemla A, Fischer D, Rychlewski L, Elofsson A. A study of quality measures for protein threading models. BMC Bioinformatics. 2001;2:5. [ Links ]
31. Bowie JU, Luthy R, Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure. Science. 1991;253(5016):164-70. [ Links ]
32. Luthy R, Bowie JU, Eisenberg D. Assessment of protein models with three-dimensional profiles. Nature. 1992;356(6364):83-5. [ Links ]
33. Ghosh S, Nie A, An J, Huang Z. Structure-based virtual screening of chemical libraries for drug discovery. Curr Opin Chem Biol. 2006;10(3):194-202. [ Links ]