Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de la Universidad Industrial de Santander. Salud
Print version ISSN 0121-0807
Rev. Univ. Ind. Santander. Salud vol.46 no.1 Bucaramanga Jan./Apr. 2014
Comparison of the ATB® Fungus 2
with the AFST-EUCAST for in vitro
susceptibility testing of Candida spp.
Jehidys Estella Montiel Ramos1, Andrea Corrales Bernal1, María Paulina Vélez Bravo1, Armando Baena Zapata1,
Ana Cecilia Mesa Arango1
1. Grupo Infección y Cáncer, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
Correspondencia: Jehidys Estella Montiel Ramos, Microbióloga y Bioanalista. Dirección: Carrera 51D No 62-29 Laboratorio 283.
Correo electrónico: jeyito126@gmail.com Teléfono: +57(4) 219 6585
Recibido: agosto 22 de 2013 Aceptado: diciembre 16 de 2013
Forma de citar: Montiel Ramos JE, Corrales Bernal A, Vélez Bravo MP, Baena Zapata A, Mesa Arango AC. Comparación de las técnicas de susceptibilidad in vitro para Candida spp. ATB® FUNGUS 2 y AFST-EUCAST. rev.univ.ind.santander.salud 2104; 46 (1): 7-14.
ABSTRACT
The increase of diseases caused by Candida spp., and the treatment failures, has underscored the need for testing the susceptibilities to antifungal agents. The commercial panel ATB® Fungus 2 was compared with the reference testing method of the European Subcommittee on Antifungal Susceptibility Testing of the Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST) for the evaluation of the susceptibility of isolates of Candida spp. to three agents. The percentage of agreement was calculated based on the minimum inhibitory concentrations. There was a high correlation for AMB (100% қ = 1.0 Bhapkar coefficient p = 1.0); while it was lower with azoles (85%, қ = 0.41, p = Bhapkar coefficient 0.02 and 83.0%, қ = 0.15, Bhapkar coefficient p = 0.0006, respectively). The ATB® Fungus 2 and AFST-EUCAST are fully comparable methods for testing the susceptibility to AMB and to lesser extend comparable for ITR and FCA.
Keywords: antifungal susceptibility, Candida spp., ATB® Fungus 2, AFST-EUCAST
Comparación de las técnicas de susceptibilidad in vitro
para Candida spp. ATB® FUNGUS 2 y AFST-EUCAST
RESUMEN
El aumento de infecciones por Candida spp. y de las fallas en los tratamientos, suscitan la necesidad de pruebas de susceptibilidad. Se comparó la marca comercial ATB® Fungus 2 con la técnica estándar del Subcomité para las Pruebas de Sensibilidad Antifúngica de la Unión Europea, de la Sociedad de Microbiología Clínica y Enfermedades Infecciosas (AFST-EUCAST) para evaluar la susceptibilidad de aislamientos de Candida spp. a tres antifúngicos. Con base en las concentraciones inhibitorias mínimas se calculó el porcentaje de acuerdo. La concordancia para anfotericina B (AMB) fue alta (100% қ = 1.0, Coeficiente de Bhapkarp = 1.0); para itraconazol (ITR) y fluconazol (FCA) fue inferior (85% қ = 0.41, Coeficiente de Bhapkarp =0.02 y 83.0 %, қ = 0.15, Coeficiente de Bhapkarp = 0.0006, respectivamente). Por lo tanto, ambas técnicas son comparables para la evaluación de la susceptibilidad a AMB; con los azoles el porcentaje de acuerdo es menor.
Palabras clave: susceptibilidad antifúngica, Candida spp., ATB® Fungus 2, AFST-EUCAST.
INTRODUCTION
The incidence of diseases caused by various fungal pathogens has increased dramatically over the past few decades being Candida spp. the most common pathogen involved in these diseases. This yeast causes a variety of diseases ranging from localized forms ofonychomycosis, vulvovaginitis andoropharyngeal candidiasis, to serious infections such asfungaemia and disseminated candidiasis, particularly, in immuno compromised patients1, 2. After the release of the first triazole antifungal and the lipid AMB formulations, several new antifungal drugs have become available. However, the increasing number of fungal infections and treatment failures in patients receiving long-term antifungal therapy has underscored the need for testing the susceptibilities of fungal pathogens to antifungal agents to guide the use of more appropriate therapies1-3. The Clinical and Laboratory Standards Institute (CLSI) and The European Subcommittee on Antifungal Susceptibility Testing of the Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST) have developed the reference broth dilution methods M27-A34 and AFST-EUCAST5 for drug susceptibility testing of Candida spp. respectively. Unfortunately, the use of these reference methods is limited because they are time-consuming and labor-intensive6. A more rapid, reliable, reproducible, and locally available antifungal drug susceptibility testing method is desirable for routine application in clinical laboratories7. Different methods such as the ATB® Fungus 2 (BioMeriéux, Marcy-l'Etoile, France) have been commercially introduced. ATB® Fungus 2 is a visible read panel designed to evaluate the susceptibility of Candida spp. and Cryptococcus neoformans to some of the most used antifungal compounds such as, AMB; 5 fluorocitosine, (5FC); fluconazole, (FCA) and itraconazole, (ITR). The aim of this study was to compare the performance of commercial panel ATB® Fungus 2 and the reference testing method AFST-EUCAST for the evaluation of the susceptibility of Candida spp. clinical isolates to ITR, FCA and AMB.
MATERIALS AND METHODS
A total of 100 clinical isolates of Candida spp. obtained from individuals attending the Laboratory of Mycology of the School of Medicine at the University of Antioquia; The infectology laboratory of the University Hospital San Vicente de Paul and the clinical laboratory of Las Americas Clinic in Medellin, Colombia, were included in this study. Candida albicans isolates were identified by germ tube induction, growth at 42°C and chlamydospores formation on cornmeal agar (Beckton Dickinson, New Jersey, USA). Other Candida species were identified by the commercial test for carbohydrate assimilation API-20CAUX (BioMérieux, l'Etoile, France). Candida parapsilosis (ATCC-22019) and Candida krusei (ATCC-6258) strains were evaluated with ITR and AMB as quality control.
The commercial kit ATB® Fungus 2 was used following manufacturer instructions closely. The kit consists of 16 pairs of cupules; one does not contain any antifungal agent (growth control) and the other pairs contain antifungal compounds previously prepared at the following concentrations: FCA (0.25μg/mL -128μg/ mL), ITR (0.5μg/mL-16μg/mL ) and AMB (0.5μg/ mL -16μg/mL). Samples were resuspended in 5mL of API®NaCl 0.85% medium (BioMeriéux, Marcyl'Etoile, France) to obtain a turbidity equivalent to 2 McFarland standard, and then 20μL of diluted sample was transferred into an ampule of ATB® Fungus 2 medium. One hundred thirty five μL of this suspension were inoculated into each cupule, and finally incubated at 35oC for 24 hours.
Minimum inhibitory concentrations (MICs) were visually determined independently by 3 previously trained readers, comparing the growth of each cupule with the samples, with the growth in the control cupule and informed as follows: 4 = no reduction in growth, 3 = slight reduction in growth, 2 = distinct reduction in growth, 1 = very weak growth, 0 = no growth. For AMB, the MIC corresponded to the lowest concentration producing complete growth inhibition (score "0"). Since there is the possibility of trailing for ITR and FCA, the MICs for these antifungal corresponded to the lowest concentration with which a score of 2, 1 or 0 was obtained. The categorical classification of susceptibility by the ATB® Fungus 2 panel was carried out using the same CLSI M27-A2 MIC breakpoint categories according to the manufacturer's instructions. A strain was considered susceptible (S) for ITR and FCA if the MICs were ≤ 0.125 μg/mL and ≤ 8μg/mL, susceptible intermediate (SI), if MICs were 0.25-0.50 μg/mL and 16-32μg/mL, and resistant (R) if MICs were ≥ 1 μg/ mL and ≥64μg/mL, respectively. These values are not formally defined for AMB by CLSI M27-A2 method, hence, we considered a strain S to this antifungal, if MICs were ≤2μg/mL, and R if MICs were >2μg/mL. Susceptibility testing for each isolate was conducted in triplicate and performed on three separate days.
For the AFST-EUCAST method, standards at the same concentrations with powders of ITR (Sigma Chemical Co, MO, USA), AMB (Sigma Chemical Co, MO, USA) and FCA (Pfizer Pharmaceutical Group New York, NY, USA) were prepared. The inocula for microdilution plates were 0.5-2.5 x 105 CFU/mL. Minimum inhibitory concentrations were determined with a spectrophotometer (Bio-Rad, Hercules, USA) at 450 nm after 24 hours of incubation at 35°C. For ITR and FCA, MICs were defined as the lowest drug concentration that inhibited 50% growth or more as compared with the control. MIC for AMB was defined as the lowest concentration producing complete growth inhibition. According to breakpoint tables for interpretation of MICs (Version 6.1, valid from 2013-03-11), the AFST- EUCAST has established the following categorical breakpoints: for AMB was MICs ≤ 1μg/mL S and > 1μg/mL R. For ITR breakpoints have not defined, thereby; we used the same used for ATB® Fungus 2: for FCA: S = MICs ≤ 2μg/mL, SI 4μg/mL, and R > 4μg/mL, except for C. glabrata: S = MICs ≤ 0,002μg/mL,SI > 0,002-32μg/mL, and R >32μg/ mL5-8. Growth and sterility controls were included in each assay. All isolates were evaluated in duplicate on three different days.
The geometric mean, range and median of MICs obtained by ATB® Fungus 2 and EUCAST for each antifungal compound were calculated for each species and determined if there was any statistical difference between the median of the MICs obtained by each method by the Mann Whitney test. The overall percentage of agreement of MICs determined by ATB® Fungus 2 and AFST-EUCAST at different tolerances was also calculated. A tolerance equals to zero means that MIC values of the sample tested by both methods were identical, whereas a tolerance equals to one, two or more, means that there was agreement between the results obtained by both methods if they only differ in one, two or more MIC values respectively. The reproducibility of the results of the ATB® Fungus 2 test obtained independently by three readers was estimated using agreement percentage and Cohen's Kappa index (қ). Concordance between ATB® Fungus 2 and AFSTEUCAST methods for categorical classification of Susceptible (S), Intermediate Susceptible (SI) and Resistant (R) strains was conducted stratifying by species. Finally, an overall analysis including all the 100 clinical isolates comparing the performance or the ATB® Fungus 2 to AFST-EUCAST was conducted using the agreement percentage, қ index, and the Bhapkar coefficient of concordance9 in order to test for all the marginal homogeneity (i.e. across all the categories simultaneously). The criteria described by Fleiss10 where a қ value less than 0.40 indicates poor agreement, 0.40 to 0.75 from acceptable to good agreement and higher than 0.75 excellent agreement was used. P value ≤ 0.05, was considered significant in all tests. The R program version 2.9, (R Development Core Team (2008): a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (ISBN 3-900051-07-0) was used for all data analyses. A significance level of 0.05 was used in all tests.
RESULTS
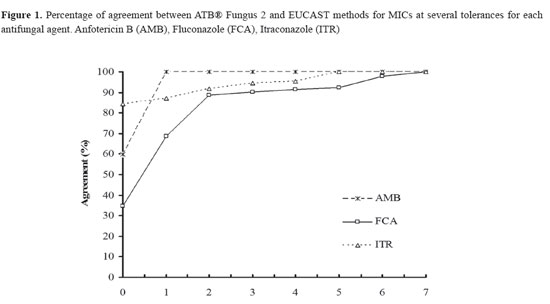
Sixty percent of the clinical isolates was C. albicans, 19% C. parapsilosis, 12% Candida tropicalis, 5% Candida glabrata, 3% Candida guilliermondii, and 1% Candida famata. Nineteen percent of the isolates were obtained from urine, 22% from nails, 14% from abdominal secretion, 13% from oral mucosa, 8% from bronchial secretion, 7% from catheters, 6% from both blood and peritoneal fluids, 11% from other samples (tongue, vaginal fluid vitreous humour, pleural fluid, ear, cyst, thigh secretion, skin secretion). The analysis of the reproducibility of the interpretation of the results of the ATB® Fungus 2 method showed that there was good agreement between the 3 independent readers for the MICs values (84-100%, κ = 0.74). Table 1 shows the geometric mean; range and median of MICs of AMB, FCA and ITR for 100 clinical isolates of Candida spp. evaluated by EUCAST and ATB® Fungus 2 methods. In order to determine percentage of agreement of MICs value between ATB® Fungus 2 and AFST-EUCAST, the numerical comparison at different tolerances was conducted. Figure 1 shows the percentage of agreement between ATB® Fungus 2 and AFST-EUCAST methods for MICs at several tolerances for each antifungal agent. At tolerance 0, the percentage of agreement for AMB was 60%, for FCA 35%, and for ITR 84%. At one tolerance, the percentage of agreement for AMB increases to 100%, for FCA at around 70% and for ITR at 87%. The only major change at 2 tolerances it is observed for FCA. The percentage of agreement for this antifungal increased to 89% with two tolerances but not major changes were observed for any of the antifungal tested to higher tolerances.
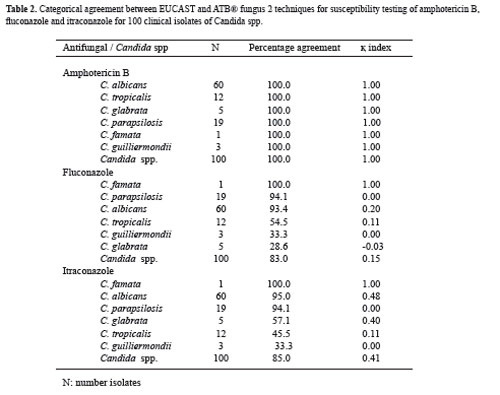
The comparison between AFST-EUCAST and ATB® Fungus 2 methods for the categorical classification of susceptibility to AMB, FCA and ITR of isolates stratified by species are shown in Table 2. The best agreement was obtained with AMB for all species included with both methods (100%; κ = 1). However the agreement with the other two antifungal (FCA and ITR) was more variable and the percentage ranged from 20 to 28.6 and the κ index from - 0.03 to 1.0, respectively. The best agreement with both azoles was observed with C. famata, C. parasilopsisand C. albicans. Nonetheless there was difference in the percentage of agreement with the species C. glabrata and C. tropicalis (Table 2).
N: number isolates
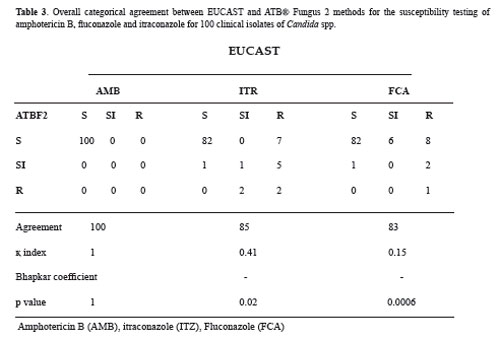
When overall categorical agreement between the two methods of susceptibility for AMB, FCA and ITR was calculated the agreement with AMB was 100 % (κ = 1.0) without significant difference between the methods when homogeneity across all the categories simultaneously was evaluated (Bhapkar test, P = 1.0) (Table 3). The categorical analysis for ITR and FCA showed percentages of agreement lower than that observed with AMB (85.0 %, κ = 0.41 and 83.0%, қ index = 0.15, respectively) (Table 3). Also it was found significant difference in the coefficient of concordance (Bhapkar test p= 0.02 and p= 0.0006, respectively) (Table 3).
DISCUSSION
Clinical laboratories need alternative methods less expensive and laborious than the standard methods for testing the susceptibility to antifungal agents6. The ATB® Fungus 2 complies with these features to test the susceptibility of Candida spp. and C. neoformansto FCA, ITR, AMB and 5FC. However the performance of this method in clinical settings has not been widely evaluated. In this study we compared this commercial kit with the reference method standard AFST-EUCAST with the antifungal most widely used in clinical practice. Evaluation of 5FC was not included in the study since wide resistance of this antifungal precludes its use as a single agent. Our results showed that the best agreement between AFST-EUCAST and ATB® Fungus 2 techniques for the categorical classification of susceptibility was observed with AMB. Unlike, the agreement between both methods with the azoles was more variable. In contrast to AMB, the azoles are fungistatic and as consequence residual growth of yeasts could occur and prevent the accurate determination of the MIC.
On the other hand, the percentage of agreement and concordance between both tests, with azoles, for categorical classification of susceptibility of the isolates was dependent on the Candida species. However, the reduced number of samples for some of the species tested limits the possibility to make a clear conclusion. The differences observed in the results with azoles could have several explanations: (i) the established cut-off points to determine the MICs are different in both techniques and the categories established for the ATB® Fungus 2 are broader than the established 50% growth inhibition of the AFST-EUCAST method. This is reflected in the fact that ATB® Fungus 2 method classified as susceptible more isolates than the AFST-EUCAST. Other plausible explanation for the differences is that there are isolates such as C. tropicalis, and C. glabrata that are subject to significant trailing growth, and that this may lead to the inaccuracy in MIC determination with the ATB® Fungus 211. This may influence the poor concordance between tests when evaluating the susceptibility of the C. tropicalis isolates (12) to ITR (45.5% and қ = 0.11) and C. glabrata (8) to FCA (28.6% and қ = -0.03), this species showed decreased sensitivity or intrinsic resistance to FCA in other studies, even ATB-F36, 12, 13.
To date, few studies have been conducted comparing the correlation between ATB® Fungus 2 and other methods. To our knowledge this is the first time that this method has been compared with the reference method AFST-EUCAST. Kantarcioglu (2001) compared the MIC values obtained by ATB® Fungus (version 1.0) with the reference method CLSI- M27A. This study did not include antifungals FCA and ITR, which does not permit comparison with our results14. More recently Torres-Rodriguez and Alvarado-Ramírez (2007), conducted a study that compared ATB® Fungus 2 with the standard CLSI M27-A2 method and Sensititre Yeast One®. They found that the overall agreements between methods were 94% and 99% for automated ATB® Fungus 2 and visual ATB® Fungus 2 readings, respectively and for Sensititre Yeast One the agreement was 91%. In this study, ITR presented the lowest concordance for Candida spp.15. Eraso et al. (2008), compared ATB® Fungus 2 with SensititreYeastOne, the agreement between methods was assessed with 133 Candida strains. The agreement (no more than two dilutions) of MIC results obtained by both methods was good (91.7-97.7%) for AMB, ITR, but lower for FCA (82.7%). On the other hand, the categorical agreement was good (93.2 to 98.5%) with 5-fluorocytosine and AMB, but lower with both triazoles (ITR and FCA) (72.9-75.9%)12. All these studies only used percentage of agreement to compare the tests, in contrast in our study κ index and Bhakpar were estimated which are more demanding than percentage of agreement.
In conclusion, our results indicate that ATB® Fungus 2 is comparable to AFST-EUCAST for the in vitro determination of susceptibility of Candida clinical isolates to AMB, and to a lesser extent to FCA and ITR. However, we suggest that minor changes in the scale to determine the MICs or categorical breakpoints may improve the performance of the ATB® Fungus 2 method to test the susceptibility to FCA. Our results are in agreement with other studies that indicate that this commercial method could be a reliable procedure to evaluate susceptibility of Candida spp. isolates, though future studies should include a higher number of other than C. albicans species and previously characterized AMB resistant isolates in order to define the utility of this kit for detect AMB resistance in vitro.
ACKNOWLEDGEMENTS
We thank Grupo Infección y Cáncer. This study was supported by Comite para el desarrollo de la investigación (CODI), Universidad de Antioquia. We also thank to BioMeriéux for the donation of ATB® Fungus 2 kits, to Laboratorio de Micología, de la Facultad de Medicina, Universidad de Antioquia and Hospital San Vicente de Paul, de Medellín for their help with collection and identification of clinical isolates.
ETHICAL CLEARANCE
This study did not need an ethics committee approval because it did not include direct contact, personal information or clinical samples of patients, just isolates.
CONFLICT OF INTERESTS
The authors have no conflict of interest to declare.
REFERENCES
1. Mishra NN, Prasad T, Sharma N, Payasi A, Prasad R, Gupta DK, et al. Pathogenicity and drug resistance in Candida albicans and other yeast species. A review. Acta Microbiol Immunol Hung 2007;54(3):201-35. [ Links ]
2. Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, et al. Guías de práctica clínica para el manejo de la candidiasis: actualización del 2009, de la Infectious Diseases Society of America. CID 2009;48:1-35. [ Links ]
3. Espinel-Ingroff A, Barchiesi F, Cuenca-Estrella M, Pfaller MA, Rinaldi M, Rodriguez-Tudela JL, et al. International and multicenter comparison of EUCAST and CLSI M27-A2 broth microdilution methods for testing susceptibilities of Candida spp. to fluconazole, itraconazole, posaconazole, and voriconazole. J Clin Microbiol 2005;43(8):3884-9. [ Links ]
4. Institute CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved standard, 3rd ed, CLSI document M27-A3200 [ Links ]
5. Rodriguez-Tudela JL, Arendrup MC, Barchiesi F, Bille J, Chryssanthou E, Cuenca-Estrella M, et al. EUCAST Definitive Document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin Microbiol Infect 2008;14:398-405. [ Links ]
6. Maldonado I, Fernandez Canigia L, Vivot W, Domecq P, Davel G, Cordoba S. [Evaluation of three methods for in vitro detection of antifungal susceptibility of Candida species]. Revista Argentina de Microbiologia 2011;43(2):120-6. [ Links ]
7. Alexander BD, Byrne TC, Smith KL, Hanson KE, Anstrom KJ, Perfect JR, et al. Comparative evaluation of Etest and sensititre yeastone panels against the Clinical and Laboratory Standards Institute M27-A2 reference broth microdilution method for testing Candida susceptibility to seven antifungal agents. J Clin Microbiol 2007;45(3):698- 706. [ Links ]
8. Testing ECoAS. Antifungal Agents. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs 2013. [ Links ]
9. Bhapkar VP. A note on the equivalence of two test criteria for hyphoteses in categorical data. Journal of the American Satistical Association 1996; 61:228-35. [ Links ]
10. Fleiss J. Statistical Methods for Rates and Proportions 1981. Wiley Interscience. [ Links ]
11. Cuenca-Estrella M, Gomez-Lopez A, Mellado E, Rodriguez-Tudela JL. Correlation between the procedure for antifungal susceptibility testing for Candida spp. of the European Committee on Antibiotic Susceptibility Testing (EUCAST) and four commercial techniques. Clin Microbiol Infect 2005;11(6):486-92. [ Links ]
12. Eraso E, Ruesga M, Villar-Vidal M, Carrillo- Munoz AJ, Espinel-Ingroff A, Quindos G. Comparative evaluation of ATB Fungus 2 and Sensititre YeastOne panels for testing in vitro Candida antifungal susceptibility. Revista Iberoam Micol 2008;25(1):3-6. [ Links ]
13. Li F, Wu L, Cao B, Zhang Y, Li X, Liu Y. Surveillance of the prevalence, antibiotic susceptibility, and genotypic characterization of invasive candidiasis in a teaching hospital in China between 2006 to 2011. BMC Infecti Dis 2013;13(1):353. [ Links ]
14. Kantarcioglu AS. Comparison of the ATB fungal method with the NCCLS broth macrodilution method (M27-A) for determining susceptibility of Candida clinical isolates. Chemotherapy 2001;13(4):446-9. [ Links ]
15. Torres-Rodriguez JM, Alvarado-Ramirez E. In vitro susceptibilities to yeasts using the ATB FUNGUS 2 method, compared with Sensititre Yeast One and standard CLSI (NCCLS) M27-A2 methods. The Journal of Antimicro Chemother 2007;60(3):658- 61. [ Links ]