Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de la Universidad Industrial de Santander. Salud
Print version ISSN 0121-0807
Rev. Univ. Ind. Santander. Salud vol.46 no.2 Bucaramanga May/Aug. 2014
Effects of low level laser therapy
and high voltage stimulation
on diabetic wound healing
María Cristina Sandoval Ortíz 1, Esperanza Herrera Villabona 1, Diana Marina Camargo Lemos 1, Rafael Castellanos1
1. Grupo de Estudio del Dolor, Universidad Industrial de Santander, Bucaramanga - Colombia.
Correspondencia: Diana Marina Camargo Lemos. Dirección: Carrera 32 No. 29-31. Correo electrónico: dcamargo@uis.edu.co. Teléfono: 634 4000 ext. 3147
Recibido: noviembre 5 de 2013 Aprobado: marzo 12 de 2014
Forma de citar: Sandoval Ortíz MC, Herrera Villabona E, Camargo Lemos DM, Castellanos R. Effects of low level laser therapy and high voltage stimulation on diabetic wound healing. Rev.univ.ind.santander.salud 2014; 46(2): 107 - 117.
ABSTRACT
Background: a review of the literature found no clinical studies in which low level laser therapy (LLLT) and high voltage pulsed current (HVPC) were compared to evaluate their effectiveness in promoting wound healing. Objective: The purpose of this study was compare the effects of LLLT, HVPC and standard wound care (SWC) on the healing of diabetic foot ulcers. Methods: randomized controlled clinical trial where diabetic patients were divided in control group (CG) treated with SWC; HVPC group received HVPC plus SWC; LLLTgroup, treated with LLLT plus SWC. HVPC was applied 45min, 100pps and 100μs. LLLTparameters were 685nm, 30mW,2J/cm2 applied to the wound edges and 1,5J/cm2 in the wound bed. All subjects were treated 16 weeks or until the wound closed. The variables were healing, healing proportion, ulcers's characteristics, protective sensation, nerve conduction studies (NCS) and quality life. ANCOVA analysis and a Fisher's exact test were applied. Results: Twenty-eight subjects completed the protocol.The healing was reached by 7/9, 8/10 and 6/9 participants of the LLLT, HVPC and CG respectively in the 16th week. There were no statistically significant differences between the groups in the healing proportion, NCS, sensory testing or quality of life (p>0,05). Conclusions: The results of this study did not demonstrated additional effects of LLL or HVPC to the standard wound care (SWC) on healing of diabetic ulcers.
Keywords: electrical stimulation, laser therapy, foot ulcers, diabetes
Efectos del láser de baja potencia y alto voltaje
sobre la cicatrización de úlceras diabéticas.
RESUMEN
Introducción: La literatura revisada no registró estudios que compararan la efectividad del láser debaja potencia (LBP) y la corriente pulsada de alto voltaje (CPAV) sobre la cicatrización de úlceras diabéticas. Objetivo: Comparar los efectos del LBP, la CPAV y el cuidado de enfermería estándar (CEE) sobre la cicatrización de úlceras diabéticas. Métodos: Ensayo clínico controlado con asignación a: grupo control (GC) tratado con CEE; (CPAV) recibió CPAV más CEE y (LBP) tratado con LBP más CEE. La CPAV se aplicó por 45min, 100pps y 100μs. Los parámetros del LBP fueron 685NM, 30mW y 2J/cm2 aplicado en los bordes de la herida y 1,5J/cm2 en los bordes de la herida. Todas las personas fueron tratadas por 16 semanas o hasta el cierre de la herida. Las variables fueron cicatrización, proporción de cicatrización, características de las úlceras, sensación protectora, estudios de conducción nerviosa (ECN) y calidad de vida. Para el análisis se aplicaron un ANCOVA y el test exacto de Fisher. Resultados: El protocolo fue completado por 28 personas. La cicatrización se logró en 7/9, 8/10 y 6/9 participantes de LBP, CPAV y CEE respectivamente, hasta la semana 16. No hubo diferencias significativas entre los grupos en la proporción de cicatrización, ECN, evaluación sensorial o calidad de vida (p>0,05). Conclusión: Los resultados de este estudio no demostraron efectos adicionales del LBP o de la CPAV, al cuidado de enfermería estándar, sobre la cicatrización de úlceras de pie diabético.
Palabras clave: estimulación eléctrica, terapia láser, úlceras de pie, diabetes.
INTRODUCTION
Diabetes mellitus (DM) is a disease in which the body does not produce or properly use insulin. This disease has increased by 100 % over the past two decades in countries like the USA and it is expected to have increased by an additional 165 % by 2050 1.
DM causes chronic complications like diabetic foot ulcers produced by multiple etiologies, including neuropathy and peripheric vascular disease. Diabetic foot ulcers often lead to deformity, infection and trauma 2. The treatment costs are high, especially due to prolonged hospitalization, rehabilitation and social relocation of the individual.
Lower extremity amputation is one of the consequences of diabetic foot ulceration; it significantly affects patient's quality of life and increases mortality by 39 % to 68 % 3. The annual incidence of lower extremity amputation varies from 5,3 to 8,1 per 1000 people with DM. The risk of lower-limb amputation is 30 to 40 times higher in the diabetic than the nondiabetic population 4.
The complexity of these ulcers requires an interdisciplinary approach, focusing on prevention, education and treatment of patients with diabetic foot ulcers; this strategy can lead to amputation being reduced by 50 % 2.
Diabetic foot ulcers can be treated by using either high voltage pulsed current (HVPC) or low level laser therapy (LLLT). High rates of healing have been reported following the treatment of several types of wound 5-17, mainly with the HVPC. This modality increases local blood flow, measured by transcutaneous oxygen pressureTcPO2, there by accelerating the healing process and promoting a bactericidal effect 8-15. Gardner 15 in one meta-analysis quantified the effect of electrical stimulation on healing diverse types of wound, concluding that such intervention produced a 22 % healing rate compared to 9 % for control wounds.
The results of using laser for treating humans are contradictory because just a few well-conducted clinical trials have been published. There are some studies confirming the efficacy of laser in healing wounds in humans 7,16, but others have not shown statistically significant differences between groups 17,18.
Among the possible LLLT mechanisms that accelerate the healing process are increase in ATP production, DNA, RNA total content, collagen synthesis and fibroblast proliferation 5. There is also evidence that LLLT increases local blood flow, thereby increasing oxygen saturation and epithelization 6. It has also been suggested that laser might improve sensory perception in patients suffering peripheral neuropathy; however the results are not conclusive 19,20.
While the available evidence supports the effectiveness of the LLLT and HVPC in enhancing wound healing, no clinical trials comparing the effectiveness of the two biophysical agents were found. The purpose of this study was to compare the additional effects of LLLT or HVPC to the standard wound care (SWC) alone on healing of diabetic foot ulcers.
MATERIALS AND METHODS
Participants
Volunteers who fulfilled the following criteria were included in the present study: 30 to 75 years old with confirmed diagnosis of DM (diagnosis based on WHO criteria), with ulcers localized on the distal legs or feet, classified as category I or II according to the Wagner Classification System. Subjects with uncontrolled diabetes, local infection in the ulcer site, ulcer grades III through V (Wagner classification), lower limb amputation and neuromuscular or musculoskeletal disease were excluded from the study. The diagnosis of DM was verified by glycemia values prior to the intervention. Each participant was examined clinically by an endocrinologist to confirm the inclusion criteria and subsequent evaluation of DM during the study.
This study was approved by the Ethics Committee of the Universidad Industrial de Santander, and all subjects signed an informed consent form. Also was registered as NCT00719251 - clinical trials.gov
Study Design
A randomized controlled clinical trial was performed. Subjects were randomly assigned to one of three groups: control group (CG) treated with standard wound care (SWC); high voltage pulsed current group (HVPC) who received HVPC plus SWC; the low level laser therapy group (LLLT), who were treated with LLLT plus SWC. The randomization process was performed according to a pre-established order, using randomized blocks.
Two physical therapists (each having 20 years of experience) conducted all the measurements; evaluators were blinded regarding group assignment. SWC was performed by a trained nurse; finally the HVPC and LLLT treatments were performed by one physical therapist.
Outcomes
Primary outcome:
Healing: defined as yes or no, if a closure of at least 75 % or more of the sum of areas of all ulcers per patient ocurred after the 16th week of intervention.
Secondary outcomes:
Healing Proportion: measurement obtained as the percent of baseline area, based on the sum of the areas of all ulcers per patient, for weeks 4, 8, 12 and 16 defined as follows: [(initial area - end area)/ initial area x 100].
Characteristics of the ulcers: Wounds were categorized using the Wagner Classification System. This system assess the wound depth, infection and ischemia and gives six wound stages. The ulcer location was identified on a picture of the foot and distal leg with views of the lateral and plantar foot. The wound surface area was measured using the acetate tracing method; this involved directly applying a clean transparent film to the wound and then tracing the wound edges. This tracing was transposed onto graph-paper and then scanned to determine wound area in cm2 using Image 1,39 usoftware (National Institutes of Health, USA). Wound depth was measured by using a sterile cottontipped applicator which was placed into the deepest part of the wound and then withdrawn and measured in millimeters. Ulcer progression toward healing was determined by surface area measurement recorded in months.
Protective Sensation Test and Nerve Conduction Studies (NCS): Peripheral nerve function was assessed by using the protective sensation testing and NCS of the sural, medial plantar and posterior tibial nerves. Data from these tests were used to show whether the therapeutic interventions induce changes in a patient's neuropathic status.
Protective sensation was examined using the Semmes- Weinstein monofilament test (Touch-Test™) which is considered a sensitive method for determining the presence or absence of protective sensation in the neuropathic diabetic foot. The monofilament test was selected and modified from Sangyeop method where a 10 g monofilament was applied only once to each of 10 points on the foot's plantar surface. However, some of the 10 points were not located within the medial plantar nerve and sural nerve's cutaneous distribution area. Sangyeop's test was thus modified by increasing the number of test points to 20, 10 points being located within the medial plantar nerve's distribution area and 10 within the sural nerve's distribution area. An abnormal protective sensation was defined as being the inability to perceive the monofilament at 6 or more evaluation sites for each nerve.
All NCS of the affected lower extremity were recorded using a Nicolet Compass Meridian™ (Madison, WI, USA). Sensory studies of the medial plantar and sural nerves were performed with a 20Hz to 3kHz band width setting, a 20 μV/division gain for the sural and 2 μV/ division for medial plantar and a 1 ms/division sweep speed. The nerve signals were obtained by averaging 20 responses evoked with 100μs rectangular pulses having amplitude adjusted to slightly more than that required to ensure a maximum response. The medial plantar nerve was studied by the orthodromic method. The nerve was stimulated through two ring electrodes placed around the hallux which was separated from the second toe by a strip of plastic sheeting. The recording electrode was placed over a point at which the posterior tibial artery could be palpated, close to the medial malleolus of the ankle. Antidromic NCS for the sural nerve was performed by placing the recording electrode just behind the lateral malleolus. The stimulating electrode was about 14cm proximal to the active recording electrode, immediately lateral to the midline of the calf muscle. The ground electrode was placed on the calf muscle.
Motor studies were obtained with a bandwidth setting of 2Hz to 10kHz, sensitivity of 2mV/div, and sweep speed of 2ms/div.These settings were modified in case of abnormal NCS. Motor fibers of the posterior tibial nerve were tested by employing the belly-tendon method. The recording electrode was placed over the abductor hallucis muscle and the reference electrode at the base of the hallux. The ground electrode was positioned on the calf muscle. The distal stimulation site was at the ankle, just behind the medial malleolus. The proximal stimulation site was at the knee, just medial to the midpoint of the knee crease.
Abnormal NCS was established as follows: <34,68m/s for the sural nerve, <40,63m/s for the posterior tibial and <24,4m/s for the plantar nerve. When compound action potential was not detected, the NCS was classified as being absent.
Quality of life
Euroquol - 5D (EQ-5D) self-report questionnaire was used; this is a generic measurement of health-related quality of life that generates a single index value based on five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). Each dimension has three response categories identified as: no problem, some problem and extreme problem. A participant can also assess his/her own overall daily health status on the EQ-5D on a 0-100 scale (EQ VAS).
Explanatory variables
Evaluation time: Wound characteristics were evaluated at the beginning of the study, prior to debridement, every two weeks during the intervention, at the end of the treatment (16th week), 30 and 60 days after the protocol had been completed. Monofilament testing, NCS and quality of life were performed at the beginning and end of the treatment.
Interventions
Standard Wound Care (SWC): This treatment was performed according to nursing intervention classification (NIC) recommendations. The procedure included irrigation with physiological saline solution, selective sharp debridement of necrotic tissue and maintaining a moist environment by applying an appropriate wound dressing. The patients were also taught diabetic foot self-care and pressure off-loading in the affected foot. All patients received SWC seven days a week for 16 weeks or until wound closure ocurred. Three days per week the patients attended the laboratory for SWC and their respective treatment (HVPC or LLLT), while on the other four days the nurse visited subjects at home to apply SWC.
High Voltage Pulsed Current (HVPC): wounds were treated with HVPC for 45 min three times a week for 16 weeks or until the wound closed. The treatment electrode was made of heavy-duty aluminum foil which was directly secured on steril saline-moist gauze loosely packed into the wound. The non-treatment (dispersive) electrode was applied 5cm from the wound edge on the same skin surface. The electrical stimulator used (Intelect 340 Stim model- Chattanooga Group) produced a twin peak pulse having the following parameters: continuous mode, submotor voltage level, 100 pulses per second (pps) pulse frequency and 100μs pulse duration. Active electrode polarity was negative (cathod) during the first three treatment sesions and then changed to positive (anode) until the end of the treatment 21. Prior to beginning the study, the electrical stimulator was calibrated with an oscilloscope (Tektronix Inc.- USA).
Low level laser theray (LLLT): semiconductor laser diode (DMC - Brazil) with 685 nm wavelength emitted 30 mW in continuous mode, 0,0028cm2 beam area applied punctually at 2J/cm2 (0,18s) every centimeter along the edges of the ulcer in light contact and 1,5J/ cm2 (0,14s) in the wound bed in non-contact mode, three times a week for 16 weeks or until the wound closed. Regarding energy density, a 3 to 5 J/cm2 dose has provided better wound healing results 16,22,23. One study concluded that high doses of photoenergy are very aggressive and may therefore decrease cellular proliferation and healing rate 5, thereby corroborating the dose-dependency effect of laser light, previously demonstrated in in- vitro studies 23. The laser probe was cleaned with a disinfectant prior to use; the laser was also calibrated with a research radiometer (International Light Technologies) prior to the study.
Data Analysis
The results followed the Consolidated Standards of Reporting Trials (CONSORT) recommendations for RCTs. The subjects were compared at the baseline on demographic and clinical characteristics, wound characteristics, protective sensation and NCS for sural, medial plantar and posterior tibial nerves, by an analysis of variance (ANOVA), Kruskal-Wallis or chi-square tests, depending on the distribution and measurement scale of each variable. The results are presented in means and standard deviations (SD), medians and interquartilic ranges (IQR) or proportions.
Analysis of covariance (ANCOVA) was used for evaluating differences by intervention group in the secondary outcomes, using the last measurement (16th week) as dependent variable and adjusting for the baseline measurement. Prior to ANCOVA, the Shapiro- Wilk test was performed to determine the normality of the data. When the asumption of normality was not fullfilled, a robust regression analysis was applied.
The comparisons for the protective sensation abnormality and the dimensions of quality of life, between the baseline and the last measurement (16th week) for each intervention group, were evaluated using a McNemar χ2 test and the sign test respectively. Healing at the 16th week, between the three intervention groups, was evaluated using a Fisher's exact test. STATA version 9,0 software was used for the analysis and P≤ 0,05 was considered significant.
RESULTS
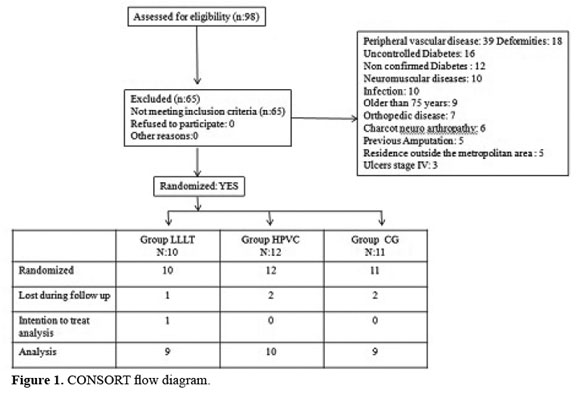
The CONSORT statement's instructions and lists of controls were followed in this randomized clinical trial. A total of 98 individuals were evaluated for participation in the study; 65 subjects did not meet the inclusion criteria and five individuals pulled out the study for reasons unrelated to the treatment. Intention to treat analysis was applied to one participant. Finally twenty-eight patients complied with the study protocol LLLT (n=9), HVPC (n=10) and CG (n=9), (Figure 1), mean age 59,3 ± 11,8 years, mean duration of diabetes 11,2 ± 10,1 years and 42,9% male. Ulcers duration 16,2 ± 34,6 months and a total of 42 ulcers distributed by intervention group 14, 15 and 13 ulcers for LLLT, HVPC and CG respectively.
Clinical baseline characteristics
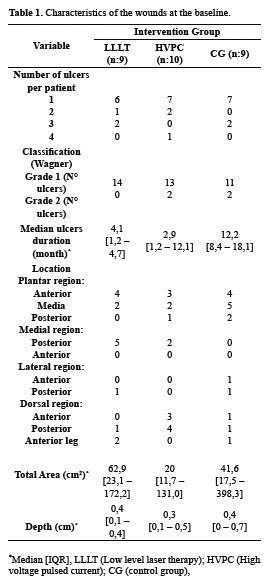
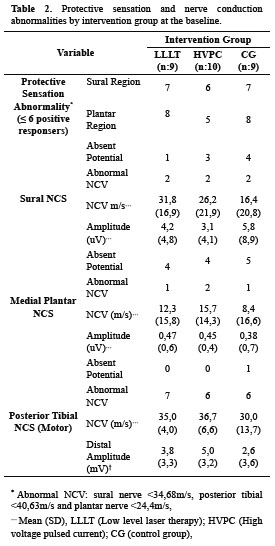
There were no significant differences between intervention groups regarding baseline variables or wound characteristics (Table 1). Protective sensation in plantar nerve distribution was altered in 75% of the subjects (21/28) and 71% of them (20/28) for the sural nerve. The following records of NCS were absent: 1 for posterior tibial, 8 for sural nerve and 13 for plantar nerve; the NCV values were abnormal for the same nerves in 19, 6 and 4 subjects, respectively (Table 2).
EuroQOL-5D baseline results showed a moderate level of overall health status self perception on a EQ VAS (0-100 scale), mean 67,8±18,6 for LLLT, 79,9±27,1 for HVPC and for CG 61,1±28,9. None of the individuals registered extreme problems, and all subjects reported to have "some problems" health-related for some of dimensions assessed.
Primary outcomes
Healing was achieved by 7/9 (77,7%), 8/10 (80%) and 6/9 (66%) participants with LLLT, HVPC and CG, respectively, during the 16 weeks of intervention.We did not find significant differences between the three intervention groups (p=0,87).
Two patients (1/LLLT and 1/HVPC) could not be evaluated at the end of the 60 days because they did not attend the last evaluation. The extent of 30 and 60 days was maintained indicating that no wounds underwent regression.
Secondary outcomes
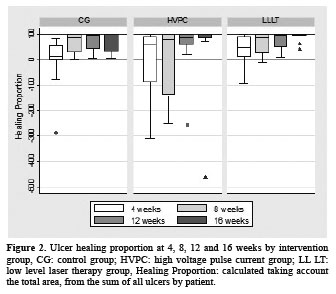
The healing proportion in weeks 4, 8, 12 and 16 increased in all groups, most subjects (21/28) reaching 75% or more closure during the 16 weeks, however the HVPC group showed higher dispersion of data at 4th and 8th weeks (Figure 2). LLLT and HPVC were closer to 100% of healing with less dispersion at 16th week.
No significant differences between intervention groups for the healing proportion at 16th week (LLLT p=0,40, HVPC p=0,36), compared to CG were found; data was adjusted for healing proportion at the 4th week.
There were no significant differences found for the abnormal protective sensation between the two intervention groups for either of the nerves tested (p>0,52).
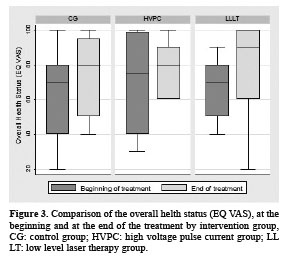
No significant differences were found for the NCS parameters studies between the intervention groups (p>0,11) for either tibial motor, plantar or sural nerves. The quality of life with EQ VAS did not demonstrate significant differences between the three intervention groups (ANCOVA p>0,18, Figure 3). Only when the sign test analysis was done, a significant difference was detected between mobility at the baseline and after the 6th week of intervention for the LLLT group (p=0,016).
DISCUSSION
The pathophysiology of DM is likely to cause a delay in the healing process and therefore increases the wound closure time, worseing the quality of the healed tissue. Some factors that contribute to delaying diabetic foot ulcer healing are inhibition of the inflammatory response, angiogenesis, fibroplasia and alterations in extracellular matrix differentiation and collagen deposition 24. Also poor nutritional support for cells, senescent fibroblasts being produced and decreased proliferative response to growth factors. Experimental studies in animal diabetic models, have reported that the initial healing phase has a slow beginning and tends to last longer, mainly due to a lower neutrophil density 25. Also, Schindl et al.16 reported that the ulcers produced by diverse causes healed faster than those caused by diabetes.
To the authors knowledge, no human studies have been conducted that compare LLLT and HVPC in diabetic ulcers treatment. Demir et. al. 26 conducted a study in healthy rats for comparing the effects of electrical stimulation and laser treatment on wound healing; the authors determined that laser light and electrical stimulation were significantly more effective when compared to control groups but the HVPC produced more beneficial effects during the inflammatory phase of healing compared to LLLT. The treatment parameters selected in this animal model differ from those selected for the current human study.
HVPC efficacy has been evaluated in conservatively treated patients with chronic wounds as diabetic foot, venous leg and pressure ulcers. However the methodology of its application has not been determined to date and differences methodological difficult the comparing of studies 27. In the current study, the HVPC parameters were selected based upon previous studies results 8-15 that support effectiveness of this physical agent on different types of ulcers. The HVPC treatment was delivered three times a week, during 45 min. Previous publications have reported positive healing outcomes following HVPC treatment protocols 5 to 7 days per week 9,12,14. Negative polarity was applied during the first three treatment sessions, given the benefits reported regarding the effect of electrotaxis on neutrophils9,10,13,15 during the inflammatory phase of healing. The cathode was subsequently applied to the wound to enhance macrophage motility and epithelial cell migration.
Franek et al. 28 examined the efficacy of high voltage stimulation for healing of venous leg ulcers in surgically and conservatively treated patients and they concluded that it is an efficient method of enhancing healing in conservative treatment of this clinical condition. Similar results were determined in the treatment of recalcitrant pressure ulcers and pressure ulcers 29,30. Clinical studies 10,12 have shown that HVPC improves blood flow and inhibits bacterial growth. Other reports and reviews have described electrotaxic effect stimulation on enhancing epithelial cells, fibroblast, neutrophil and macrophage motility 31. Nevertheless, the latter effect may occur because the HVPC signal mimics the endogenous electric fields existing in wound tissue. Physiologically, a measureable endogenous injury current has been shown to influence cellular and molecular action contributing to wound closure and healing. Such injury current continues to enhance wound repair until closure of the wound is achieved. The exogenous application of electrical stimuli in chronic wounds, such as diabetic foot ulcers, may activate healing mechanisms by restoring or enhancing an injury's endogenous current31.
Since the laser light started being used in the clinical setting, it has been considered a potential source of photoenergy for stimulating wound healing 5,6. However, methodologic problems in laser light research (i.e. irradiation protocols, variations in cell culture models, the animals species studied and the types of wounds) have hampered laser studies being compared, some of which are contradictory 6,7,16-18,32. Studies on cell cultures and animal models (mice and rats), support the use of laser on wounds 22,23,26,32-34. Only a few case studies and any clinical trials have been conducted on humans, with controversial results.
Taradaj et al. 35 studied the effectivity of LLLT in healing of venous leg ulcers and concluded that the application of this treatment does not enhance healing of venous ulcers in surgically and conservatively treated patients. In other research 36 was assessed the efficacy of laser therapy at different wavelengths: 940, 808, and 658 nm for treating pressure ulcers. The primary endpoint in this trial included both the percentage reduction of the ulcer surface area and the percentage of completely healed wounds after one month of therapy (ulcer healing rate). The secondary endpoint was the ulcer healing rate at the follow-up evaluation (3 months after the end of the study). In total, 72 patients with stage II and III pressure ulcers received laser therapy once daily, 5 times per week for 1 month using a diode laser with a maximum output power of 50mW and continuous radiation emission. An average dose of 4 J/cm2 was applied. The laser therapy at a wavelength of 658nm appeared to be effective at healing pressure ulcers. The wavelengths of 808 and 940 nm did not have any effect in this study. A review concluded that laser in humans does not improve wound healing in pressure, venous or post-surgery ulcers37. The results of other previous studies have shown that there were no differences between the laser group and sham or control group 17,18,38.
Several hypotheses have addressed LLLT action mechanism on wound healing. Kawalec et al. 5, have proposed that photochemical stimulation of atoms or molecules by laser light, increases cellular function, macrophage migration and cytokine secretion. In Schindl et.al.6, clinical trial using LLLT on diabetic patients demonstrated increase in the skin microcirculation which may have ocurred secondary to the liberation of substances which stimulated endothelial cell proliferation during angiogenesis 39, 40.
Others studies 32,33 have reported that laser light increases collagen synthesis and improves biomechanical and biochemical properties of injured tissues. Silveira et.al.22, demostrated that LLLT improves wound healing and significantly increases mitochondrial respiratory chain complex II and IV activity. Their study confirmed Karu's results 23, which suggested that cytochrome C oxidase (complex IV) might be activated by LLLT; this fact supports the previously described biostimulantion effect.
The NCS data confirmed a greater conduction loss in the sensory fibers of the medial plantar nerve. 50 % of the study population also exhibit altered NCV of the motor component of the tibial nerve; this condition shows an advanced neuropathic state. The results of monofilament testing showed abnormal protective sensation in plantar and sural nerve cutaneous distribution.
Previous research 19,20 has presented conflicting results regarding LLLT effects on NCS parameters. Prendergast et. al. 19, applied monocromatic infrared photoenergy to the lower extremities of patients diagnosed with diabetic neuropathy. The conclusion is that this treatment was partially effective on restoring distal sensory perception. Zinman et. al. 20, assesed LLLT effect on sensory and motor deficits in diabetic polyneuropathy patients, no electrophysiological or sensory perception changes were found. In the same direction, in the current study neither one of the two biophysical agents affected NCS and/or protective sensation.We assume that the no treatment effect on the neuropathy condition may have been mainly due to evolution time of the disease (11,2±10,1 years) and the subjects' age (59,3 ±11,8 years), from this two factors it can be concluded an advanced state of neuropathy, for which the interventions could not were effective.
Regarding the EQ-5D dimensions, in spite of there was no difference in overall health status with EQ VAS, individual dimension analysis revealed that mobility dimension improved with LLLT treatment, no changes were observed for the other two groups.
The results did not show any difference between the three intervention groups for any of the analyzed variables. Our results are an important advance for the scientific support of biophysical agents (laser and high voltage current) use in the clinical setting, since a few studies in humans are available. Besides, no controlled clinical studies comparing two biophysical agents' effectiveness had been conducted. The small sample size limits this study for evaluating the differences between the two methods and may have decreased the likelihood of detecting differences between intervention groups for primary and secondary outcomes. In future studies we recommend a greater sample size as well as the inclusion of additional patient care components, such as weight off-loading, type of footwear, nutritional control and education related to DM and its care for both patients and their families.
In conclusion the results of this study did not demonstrate additional effects of LLLT or HVPC to the standard wound care (SWC) on healing of diabetic foot ulcers. Also no changes were identified in the protective sensation, NCS or the quality of life of patients.
FINANCING
This project was funded by COLCIENCIAS.
ETHICAL CONSIDERATIONS
This study was approved by the Ethics Committee of the Universidad Industrial de Santander, and all subjects signed an informed consent form.
INTEREST CONFLICTS
The authors declare do not have any interest conflict with participants or entities that contributed to this study.
IN MEMORIAM
To Professor Maria Cristina Sandoval Ortiz, for her dedication and scientific contributions to the Physical Therapy field, devoted much of her research to the study of the electro-thermal modalities effects, this being the last manuscript published by her in the field. Rest In Peace.
REFERENCES
1. McBean M, Li S, Gilberston DT, Collins A. Differences in diabetes prevalence, incidence, and mortality among the elderly of four racial/ ethnic groups: whites, blacks, hispanics and asians. Diabetes Care 2004; 27:2317-24. [ Links ]
2. Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot?. Diabetes Metab Res Rev 2000; 16:75-83. [ Links ]
3. Brem H, Sheehan P, Boulton AJM. Protocol for treatment of diabetic foot ulcers. Am J Sur 2004; 187:1S - 10S. [ Links ]
4. Ramsey SD, Newton K, Blough D. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999; 22:382-7. [ Links ]
5. Kawalec JS, Pfennigwerth TC, Hetherington VJ, Logan JS. A review of lasers in healing diabetic ulcers. Foot 2004; 14:68-71. [ Links ]
6. Schindl A, Schindl M, Schon H. Low intensity laser irradiation improves skin circulation in patients with diabetic microangiopathy. Diabetes Care 1998;21:580-4. [ Links ]
7. Kazemi-Khoo N. Successful treatment of diabetic foot ulcers with low-level laser therapy. Foot 2006;16:184-7. [ Links ]
8. Kloth L, Feedar J. Aceleration of wound healing with high voltage monophasic pulsed current. Phys Ther 1988; 68:503-8. [ Links ]
9. Baker LL,Chambers R, Demuth SK, Villar F. Effects of electrical stimulation on wound healing in patients with diabetic ulcers. Diabetes Care 1997; 20:405- 12. [ Links ]
10. Peters EJG, Armstrong DG, Wunderlich RP, Bosma J. The benefit of electrical stimulation to enhance perfusion in persons with diabetes mellitus. J Foot Ankle Surg 1998; 37:396-400. [ Links ]
11. Goldman RJ, Brewley BI, Golden MA. Electrotherapy reoxygenates inframalleolar ischemic wounds on diabetic patients. A case series. Adv Skin Wound Care 2002; 15:112-20. [ Links ]
12. Goldman R, Rosen M, Brewley B, Golden M. Electrotherapy promotes healing and microcirculation of infrapopliteal ischemic wounds: a prospective pilot study. Adv Skin Wound Care 2004; 17:284 -94. [ Links ]
13. Peters EJG, Lavery LA, Armstrong DG, Fleischli JG. Electric stimulation as an adjunct to heal diabetic foot ulcers: A randomized clinical trial. Arch Phys Med Rehabil 2001; 82:721-5. [ Links ]
14. Houghton PE, Kincaid CB, Lovell M, Campbell KE. Effect of electrical stimulation on chronic leg ulcer size and appearance. Phys Ther 2003; 83:17- 28. [ Links ]
15. Gardner SE, Frantz RA, Schmidt FL. Effect of electrical stimulation on chronic wound healing: a meta-analysis. Wound Repair Regen 1999; 7:495- 503. [ Links ]
16. Schindl M, Kerschan K, Schindl A, Schön H, Heinzl H, Schindl L. Induction of complete wound by lowintensity laser irradiation depends on ulcer cause and size. Photodermatol Photoimmunol Photomed 1999; 15:18-21. [ Links ]
17. Lucas C, Van Gemert MJ, de Haan RJ. Efficacy of low level laser therapy in the management of stage III decubitus ulcers: a prospective, observer-blinded multicenter randomized clinical trial. Laser Med Sci 2003;18:72-7. [ Links ]
18. Kopera D, Kokol L, Berger C, Hass J. Low level laser: does it influence wound healing in venous leg ulcers: a randomized, placebo-controlled, doubleblind study. Br J Dermatol 2005; 152:1368-70. [ Links ]
19. Prendergast JJ, Niranda G, Sánchez M. Improvement of sensory impairment in patients with peripheral neuropathy. Endocr Pract 2004; 10:24-30. [ Links ]
20. Zinman LH, Ngo M, Ng E, New KT, Nwe KT, Gogov S, Bril V. Low-intensity laser therapy for painful symptoms of diabetic sensorimotor polyneuropathy. Diabetes Care 2004; 27:921-4. [ Links ]
21. Mertz PM, Davis SC, Cazzaniga AL, Cheng K. Electrical stimulation: acceleration of soft tissue repair by varing the polarity. Wounds 1993; 5:153-9. [ Links ]
22. Silveira PC, Streck EL, Pinho RA. Evaluation of mitochondrial respiratory chain activity in wound healing by low-level laser therapy. J Photochem Photobiol B 2007; 86:279-82. [ Links ]
23. Karu TI. Chapter 48: Low-Power laser therapy, in: Biomedical Photonics Handbook. Tuan Vo-Dinh (Editor in chief). Boca Ratón: CRC Press, 2008. [ Links ]
24. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005: 366:1736-43. [ Links ]
25. Komesu MC,Tanga MB, Buttros, KR, Nakao C. Effects of acute diabetes on rat cutaneous wound healing. Pathophysiology 2004;11:63-67. [ Links ]
26. Demir H, Balay H, Kirnap M. A comparative study of the effects of electrical stimulation and laser treatment on experimental wound healing in rats. JRRD 2004; 41:147-54. [ Links ]
27. Polak, A., Franek, A., & Taradaj, J. High-Voltage Pulsed Current Electrical Stimulation in Wound Treatment. Adv Wound Care 2014; 3: 104-117. [ Links ]
28. Franek A, Taradaj J, Polak A, Cierpka L, Blaszczak E. Efficacy of high voltage stimulation for healing of venous leg ulcers in surgically and conservatively treated patients. Phlebologie 2006; 34:255-261. [ Links ]
29. Franek, A., Kostur, R., Polak, A., Taradaj, J., Szlachta, Z., Blaszczak, E., et al. Using highvoltage electrical stimulation in the treatment of recalcitrant pressure ulcers: results of a randomized, controlled clinical study. Ostomy Wound Manag 2012; 58: 30-44. [ Links ]
30. Franek A, Kostur R, Taradaj J, Blaszczak E, Szlachta Z, Dolibog P, Polak A. Effect of High Voltage Monophasic Stimulation on Pressure Ulcer Healing: Results From a Randomized Controlled Trial . Wounds 2011;23:15-23 [ Links ]
31. McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: Current views and future potential. Physiol Rev 2005; 85:943-78. [ Links ]
32. Reddy GK. Comparison of the photostimulatory effects of visible He-Ne and infrared Ga-As lasers on healing impaired diabetic rat wounds. Lasers Surg Med 2003;33:344-51. [ Links ]
33. Maiya GA, Kumar P, Rao L. Effect of low intensity Helium-Neon (He-Ne) laser irradiation on diabetic wound healing dynamics. Photomed Laser Surg 2005; 23:187-190. [ Links ]
34. Al-Watban FAH, Zhang XY, Andres BL. Low- Level Laser Therapy enhances wound healing in diabetic rats: a comparison of different lasers. Photomed Laser Surg 2007; 25: 72-7. [ Links ]
35. Taradaj J, Franek A, Cierpka L et al. Failure of low-level laser therapy to boost healing of venous leg ulcers in surgically and conservatively treated patients. Phlebologie 2008; 37: 241-246. [ Links ]
36. Taradaj J, Halski T, Kucharzewski M, et al. Effect of laser irradiation at different wavelengths (940, 808, and 658 nm) on pressure ulcer healing: results from a clinical study. Evidence-Based Complementary and Alternative Medicine, 2013; Article ID 960240, 8 pages. [ Links ]
37. Freanek A, Krol P, Kucharzewski M. Does low output laser stimulation enhance the healing of crural ulceration? Some critical remarks. Med Eng Phys 2002; 24:607 - 15. [ Links ]
38. Sobanko JF, Alster TS. Efficacy of low-level laser therapy for chronic cutaneous ulceration in humans: a review and discussion. Dermatol Surg 2008; 34:991-1000. [ Links ]
39. Kipshidze N, Nikolaychik V, Keelan MH, Shankar LR. Low-power helium-neon laser irradiation enhances production of VEGF and promotes growth of endothelial cells in vitro. Lasers Surg Med 2001; 28:355-64. [ Links ]
40. Corazza AV, Jorge J, Kurachi C, Bagnato VS. Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources. Photomed Laser Surg 2007; 25:102-106. [ Links ]