Introduction
In Uruguay, a large proportion of agriculture uses genetically modified (GM) varieties. Soybean is the primary extensive crop in Uruguay and has been since 2004. It covers over 1 million hectares, nearly all of which is planted with GM crops. A large percentage of corn in the country is also grown with GM seeds.
Although no official data exist, 2016 and 2017 annual reports by the ISAAA (International Service for the Acquisition of Agri-biotech Applications) indicate the magnitude to which this technology has been adopted for both crops, as well as its continued growth. The percentages of soy and corn crops that have been planted with GM seeds were 97.6% and 85.7 % in 20161, and 98% and 100% in 20172, respectively.
The adoption of this agricultural production profile is similar to other Latin American countries (Argentina, Brazil, Paraguay, and Bolivia). Moreover, several sources have reported that the greatest expansion of GM crops has occurred in the Southern Cone of Latin American.
The introduction of genetically modified organisms (GMOs) in Uruguay began early. The planting of this type of crop was first approved in 1996 with the Monsanto company’s release of the use of soybean event GTS 40-3-4, commercially known as RR (Roundup Ready) soybean, which is tolerant to glyphosate herbicide. At that time, soy was an insignificant crop in the country, with under 10,000 hectares3, and there was no regulatory framework for the introduction of GM vegetables. The approval was the responsibility of the Department of Agricultural Protection Services, of the General Directorate of Agriculture Services at the Ministry of Agriculture, Livestock, and Fisheries (MGAP in Spanish). The Committee for Risk Analysis of Genetically Modified Materials was responsible for the risk assessment, made up of representatives from the National Institute for Food Research (INIA in Spanish), the National Seeds Institute (INASE in Spanish), and the General Directorate of Agricultural Services (DGSA in Spanish).
Next to be released for commercial use, in 2003 and 2004, were GM corn events, specifically, MON810 by the Monsanto company and Bt11 by Syngenta. MON810 is resistant to lepidopteran insects and tolerant to glyphosate and Bt11 is lepidopteran resistant and tolerant to glufosinate-ammonium herbicide.
The Commission for Risk Assessment of Genetically Modified Vegetables (CERV in Spanish) recommended the release of these two new corn events. Created in August 2000, this commission was tasked with advising the MGAP and the Ministry of the Economy and Finances (MEF in Spanish) on the evaluation, management, and communication of risks.
In the midst of strong controversy around the procedures for these releases, the National Directorate of the Environment (DINAMA in Spanish) implemented a project in 2005 to develop a National Biosafety Framework that would bring the country in line with the commitments it assumed under the Cartagena Protocol on Biosafety to the Convention on Biological Diversity. These actions led to the creation of the National Project Coordinating Committee, responsible for defining the various participating entities.
In 2007, having understood that it was impossible to simultaneously debate the creation of the National Biosafety Framework while also approving new GM events, an 18-month moratorium was established on new requests for authorization to introduce GM events for vegetables.
After a series of actions, a presidential executive order for a moratorium was issued in 2008 and a new institutional biosafety structure was created, placing the National Biosafety Cabinet (GNBio in Spanish) in charge of decision-making and resulting in the regulatory framework that is still in effect today.
This cabinet includes the MGAP, which presides, as well as the ministries of: Public Health (MSP in Spanish); Economy and Finances (MEF in Spanish); Housing, Land Planning, and the Environment (MVOTMA in Spanish); Industry, Energy, and Mining (MIEM in Spanish); and Foreign Relations (MRE in Spanish). This marked the inception of the political entity that authorizes new applications for vegetables. The Commission for Risk Management (CGR in Spanish) advises this cabinet and handles operations, and is made up of a delegate from each of the six ministries in the GNBio. In turn, the CGR is advised by another technical-scientific entity, the Commission for Biosafety Risk Assessment (ERB in Spanish), which is composed of members proposed by the CGR and designated by the GNBio, and is responsible for developing risk assessment reports.
Another group that supports the risk assessment process is made up of representatives from governmental organizations, research institutes, and the University of the Republic. This group is called the Inter-Institutional Development Committee (CAI in Spanish), and acts as a case-by-base advisor upon request by the ERB.
The executive order mentioned also provides for the participation of social organizations through two non-binding entities: the Consulting Committee on Biosafety (CCB in Spanish) and a consulting group on the release of new events.
With this new regulatory framework in force, the release of several new events has been authorized. In 2011, five corn events were released (two tolerant to glyphosate herbicide, one glyphosate tolerant and lepidopteran resistant, one tolerant to glufosinateammonium herbicide and lepidopteran resistant, and one that is lepidopteran resistant and tolerant to glyphosate and glufosinate-ammonium). In 2012, there were the approvals of three soy events (two tolerant to glufosinate-ammonium and one glyphosate tolerant and lepidopteran resistant) and three corn events (all lepidopteran resistant and tolerant to glyphosate and glufosinate).
Another soybean was released in 2014, which was tolerant to imidazolinone herbicides. And in December of 2017, four events were authorized, about which there were very opposing views at the core of the CGR, and which in a certain way reflected highly disparate positions among the members of this committee virtually since its outset.
The four events that were released were done so without the approval of the MSP or the MVOTMA. A lack of strong evidence regarding the safety of these foods played a role in this absence of an official statement by the country’s health authorities.
This has been a presentation of how decision-making regarding the release of GM foods has been managed in Uruguay. In this light, a question arises as to why some ministries in the country have not reached a common position on the approval of new events. To this end, it is important to mention the studies and contributions on this topic by various authors.
According to Landrigan4, GM crops can potentially produce previously unknown allergens or toxins, which could alter the nutritional quality of the food.
This conviction has led to intense debate between the supporters and opponents of the use of GM foods, in the absence of the evidence needed to characterize their risks and benefits with certainty5,6. The strong ethical and social debates revolve around their use and their ability to affect the ecosystem and human health7. With regard to human health, Hilbeck8 states that no epidemiological studies have been conducted that enable asserting that there are no risks to human health9, and that those who make that assertion do not have a scientific basis for it. For others10, it seems that GM crops do not pose a greater risk to the population’s health than traditional crops.
The principal genetic modifications that have been used in food crops have resulted from the introduction of tolerance to herbicides and resistance to insect infestations.
The present work is primarily focused on reviewing the effects of using crops in which resistance to insect infestations has been introduced, particularly Cry proteins.
Genetically modifying crops to be resistant to insect infestations involves the introduction of Cry proteincoding genes. Cry proteins are highly toxic and are the primary ones to have been widely demonstrated to have insecticidal power on Bacillus thuringiensis (Bt).
The insecticidal power of this bacteria was discovered early in Japan, in 1901, and was rediscovered in Germany in 191111. This is related with the production of a group of proteins known as δ-endotoxins12. Of these, Cry proteins have the greatest virulence against several economically important insect infestations, including Lepidoptera, Coleoptera, Diptera, and Nematodes13,14.
In addition to their high specificity against various types of insects, their persistence in the environment is low. These supposed benefits drove numerous efforts that led to the isolation of several genes coding for the synthesis of these proteins with insecticidal properties, which are being inserted into various vegetables in order to keep crops healthy.11 This type of compound has led to most of the existing evidence as well as most of the concerns about the possibility of health disorders in humans.
This is why an exhaustive study is required before introducing a new food to the market15,16. Thus, the objective of this work was to review and describe the epidemiological evidence related to the consumption of insect-resistant GM foods and its possible association with harm to health or health disorders in humans.
Methodology
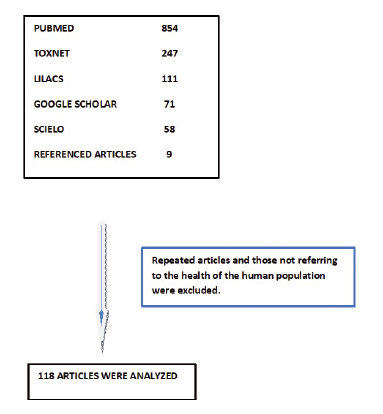
A search was performed in the databases PUBMED, SCIELO, TOXNET, and LILACS for the period 2007- 2019, with keywords “Genetically modified food,” “Genetically modified crops,” “Human Health,” “Epidemiological Research,” “Allergenicity,” “Safety,” “Health Risks,” “Bt proteins,” “Bt toxins,” and “Human cells.” Boolean operators were used. The languages included Spanish, Portuguese, and English. In order to cover the largest possible amount of articles, a search was also conducted in Google Scholar, and a snowball search was performed with the references of the articles selected.
A total of 1,350 articles were obtained (Figure 1). Repeated articles were excluded, as well as those that were not related to population health based on an epidemiological approach. A total of 118 were analyzed.
Results
Types of Actions by Cry proteins
Cry proteins are active on various orders of insects and nematodes11.
These proteins take effect when they are ingested and solubilized in the alkaline medium of the middle intestine and bind to specific receptors in the cellular epithelial of the gastrointestinal tract, inserting themselves into the membrane and generally resulting in the destruction of the intestinal epithelial17. Different models have been proposed to explain the lethal activity of Cry proteins when binding to specific receptors. The most well-known are Bravo, Zhang, and Jurat-Fuentes.12 These three models come from studies performed primarily with Lepidoptera, in which Cry proteins are particularly active, although the physiological and/or biochemical characteristics in the middle intestine of different types of insects are known to alter the process under which the Cry protein carries out its activity18.
According to Bravo et al.12, BL2 is the enzyme that facilitates the binding of Cry proteins to aminopeptidase N (APN), alkaline phosphatase (ALP), and glycolipid receptors. The absence of the BL2 enzyme in mammals is considered to be the reason why it is not toxic to them19,11.
Nevertheless, it is not clear that the binding of Cry proteins to receptors is indispensable for its developing lethal action20,21. That some Cry proteins can be toxic through other mechanisms is even accepted.
Additionally, and equally important, Cry proteins that are consumed when eating food produced with GM crops are not identical to those that naturally occur in B.thurigensis.
An important distinction is the lower specificity and greater toxicity of the gene-encoded Cry proteins that are introduced in certain crops. A good deal of research has been conducted on the mechanism of action of Cry proteins, which has generated diverse types of knowledge that is used to develop strategies to modify recombinant Cry proteins in order to broaden the spectrum of activity and/or increase lethality. By combining some of these strategies, the toxicity has increased 36 times over the original toxin22, and the specificity has decreased, expanding control over a larger number of species.
Health Effects
This could negatively affect health, and those who question introducing foods that are produced with GM crops into the diet assert that the health effects have not been adequately studied and should be exhaustively researched before they enter the market, including the possibility of allergic reactions15,23 and antibiotic resistance15.
For example, an event that has been authorized for animal consumption, such as the case of the company that created a GM corn called Star Link24, was modified with an additional gene to produce insect resistance. Information from Bacillus thuringiensis was transferred, resulting in the expression of the Cry9c protein, which demonstrated significant allergenic activity. This is why its use in the United States was approved only for animal feed24. Nevertheless, it was later found in food destined for human consumption, for example in “tacos,”15,25 a traditional food in Mexico, as well as in other corn-based products25.
Currently, when an event is approved in the United States, it is either approved for all types of consumption or it is not approved26.
The allergic reaction is generated due to a protein that is synthesized from the inserted gene27. Only one allergy has been reported, which occurred in a worker in a plant using Bacillus thuringiensis microbials11.
The Cry1Ab protein has been detected in events such as Bt11 corn19 and Mon81028, and was isolated in the gastrointestinal tract of calves fed with GM foods. Nevertheless, it was not detected in their blood19, nor were IgE antibodies detected in people who consumed this food.
And in the particular case of Bt MON810, no changes in the immune response have been found26.
It is worth noting that while many proteins are digested through the action of gastric juices,26 certain health conditions can cause people to have a higher pH, which could enable these allergens to act26.
Bioequivalence
Bioequivalence studies have been developed that involve measurements to determine whether a new product will act in a way that is similar to the original product29. Authors such as Garcia30 consider this model to be flexible, malleable, and open to interpretation.
Bioequivalence tests are recognized as valuable for determining the safety of an event to be introduced. This means demonstrating that no significant differences exist between the use of the food from the new event and the use of the original food under the same conditions and dose31,32.
Nutritional equivalence can be inferred when a comparative evaluation of the compositions does not find any significant differences between the GM plant and its appropriately-selected counterpart (the closest or most isogenic but not genetically modified). Nevertheless, when this cannot be shown, then there is a need to conduct more in-depth and adequately-designed studies33. Not all authors consider this equivalence test to be a guarantee for safety7,34,35.
The set of accumulative tests that is needed for this evaluation involves comparing the similarity of amino acid sequences (bioinformatics search), which is recognized as substantial equivalence24,27,36,37.
These tests are used to study the amount of proteins or RNA, or the total number of changes in individual transcripts or proteins that would be produced in the GMO versus its conventional counterpart38. Even with these more complex techniques, a failure to detect differences does not prove the absence of differences, and by extension, does not prove safety38. All methods have detection limits, which may limit the ability to detect and analyze the possible harm that may actually exist38.
See Heinermann (2011)38 for details about molecular profiling and what is known as Omic’s Technologies39.
In the European Union, the safety of a GM crop is partly determined using an equivalence test approach, as reported previously40. Under this paradigm, no phenotypic differences or differences in composition should exist between the GM crop and its isogenic40. These comparisons are made by cultivating the two varieties under the same conditions in the same field trial40.
This requires randomly selecting the sites where the different genotypes will be planted.40 The aim is to show that they are not different than their isogenic referent and are equivalent to others that have been used and have been proven to be safe40.
The use of new mathematical models has been proposed to measure the effect of the experiment while controlling the background41, which has to do with the natural genetic variability of crops as well as the conditions under which the experiment is performed.
These are not the only tests. Serum specific36, pepsinresistance36, and in vitro digestibility tests can also be performed39.
IgE-Mediated Responses
IgE-mediated responses can occur in human and animal models without an adverse clinical reaction. Therefore, systemic reactions need to be more thoroughly understood so as to better understand and refine approaches to evaluating safety in the future42. In vitro trials have not identified an IgE-mediated response24.
Current guidelines also include evaluating nonIgE-mediated adverse reactions (enteropathies that are not IgE-mediated, e.g. allergic eosinophilic gastroenteropathy) on a case-by-case basis43. As the number of events begin to increase, evaluations are needed to determine that allergic reactions in human and animal populations are not also increasing, on a case-by-base basis, given that addition, synergism, or antagonism could occur43.
Toxicological Evaluation
The EFSA has published guidelines for toxicological evaluations, which recommend animal feeding trials in order to characterize the risks of using GMOs and to detect possible toxicological effects of test diets compared to control diets. It has also established a protocol to follow when testing foods using 90-day repeated dose toxicity studies in rodents43, considered reliable animals for detecting undesired health effects44. Other authors30,37 have indicated the limits of these studies as well as the need for case-by-case evaluations35,37. Those who promote and conduct risk assessment studies, also suggest the need for case-bycase monitoring16.
Lastly, limitations have been highlighted regarding the ability to conclude whether or not these foods are safe, given that most of the evidence has primarily been based on in vitro and experimental animal studies. And since the latter have generally been conducted over short time periods, they cannot detect long-term effects8,26.
Epidemiological Evidence
In the absence of long-term studies, the specific hypotheses about GM foods have been analyzed by comparing epidemiological data from Canada and the United States with data from the United Kingdom and the Western European Union26, populations that are recognized as comparable. GM foods have been consumed in the United States and Canada since the mid 1990s, while they have not been as widely consumed in the United Kingdom and the Western European Union26.
The changing patterns in the incidence of cancer in the United States and Canada are very similar to those in the United Kingdom and the Western European Union.26 In addition, the available data do not support the hypothesis that the consumption of GM foods has increased the rates of obesity or type 2 diabetes, or the prevalence of chronic kidney disease in the United States26.
The detection of an increase in Celiac disease began in the United States before GM crops were introduced, and this increase seems to be similar in the United Kingdom where GM foods are not typically consumed26.
The increasing pattern in autism spectrum disorder in children in the United States and the United Kingdom does not support the hypothesis that consuming GM foods is associated with the prevalence of this disorder26.
Therefore, based on a detailed examination of shortand long-term toxicity tests in laboratory animals and the available epidemiological data, it has not been possible to demonstrate a greater risk to human health from GM foods than from conventional foods26. Given the type of evidence available, many uncertainties exist, making it difficult to extrapolate the findings to the human population.
Monitoring System
There is a need for a well-designed, long-term, postmarket monitoring system with sufficient resources4,16.
As part of this proposed monitoring system, databases on the composition of foods should be improved and maintained so that they are kept up-to-date on changes that may be identified by post-market studies24,44. Adverse health effects, such as a type of allergy, also need to be registered even if serious clinical manifestations are not44.
Based on what has already been stated, post-market monitoring systems should be case-by-case, and preferably designed before products enter the market in order to facilitate prospective observation.
Consumer Information
When mentioning the advantages of using GM foods, it is important to remember that the molecules (Cry proteins) that have been introduced act as toxins45.
One of the measures that is being promoted is food labels for products that are developed from genetic modifications. Nevertheless, companies have shown a degree of fear about the effect of labeling on their business.
Labeling is essential for monitoring, and, is important considering the possible emergence of new food allergies. This would respect the wishes of a growing number of consumers who insist on the right to know what kind of food they are buying4,7,46. Some even believe that labeling should be mandatory.47
Discussion
Most of the existing information is related with studies of chemical composition, in vitro trials, or laboratory animal trials.
Substantial equivalence is the guiding principle, which indicates equivalence in all senses. This assumes that undesired negative side effects can be detected just by performing a chemical analysis. Actually, this concept should not substitute the need to rigorously evaluate products through nutritional, immunological, and toxicological trials27, supplemented by in vivo studies48. The lack of specific receptors in mammals cannot be one of the main arguments23 for a lack of toxicity to human health. Cry proteins are known to be found in soluble form, which can damage cellular membranes48 as well as bioaccumulate48.
Complex studies are needed that reliably evaluate the effects of consuming foods produced through genetic engineering44, regardless of the idea that people are accustomed to eating processed foods and that such a diet would denaturalize these proteins, making them safer to consume49.
Another argument is that they are consumed in small quantities, but that does not take into account their possible bioaccumulation in tissues, given that they have been detected in the blood of pregnant women48, according to a study in Canada50. Although this was later refuted due to a discrepancy in the technique used to measure Cry proteins51,52. An additional step in generating scientific evidence is needed, namely, conducting epidemiological studies that can measure individual consumption44,53. This would generate the greatest amount of evidence54.
In order to determine whether or not conventional or GM foods can cause specific health and food safety problems, it is also important to better understand the impact of foods on the immune system and their interaction with it55.
Therefore, this review has relied on short- and longterm toxicity tests in animals and on the available epidemiological information. Even with all this, it has not been possible to demonstrate that GM foods present a greater health risk than conventional foods26,56.
Nevertheless, the insertion of genes is known to impact the metabolites of the plant itself26, which makes it very difficult to evaluate the safety of a food product.
One limitation of this review is its focus on a search of epidemiological studies and on evaluating the level of evidence57. This review demonstrates the existence of descriptions of isolated cases and ecological studies. No studies are available that enable being conclusive about the reported findings.
Given this uncertainty, effective communications with the population should be a priority so that consumers can choose between conventional and unconventional foods4.
Based on the studies analyzed, it is not possible to be conclusive about the safety of these foods.