I. Introduction
Energy production from waste (Waste to Energy) has gained popularity thanks to the search for solutions that are sustainable for the environment, society and economy. Energetic valorization of waste allows decreasing greenhouse emissions and waste final disposal in sanitary landfills, and increasing material retrieval. The world siderurgic industry has identified practices to rationally and efficiently use energy, which is intensively use in this industry, and to take advantage of the co-products resulted from the preparation and transformation of raw material and supplies into finished steel products. The Ministry of Environment and Sustainable Development of Colombia has developed a siderurgic NAMAS (Nationally Appropriate Mitigation Action) [1] that includes activities such as using waste to produce fuels, like the project described in this paper.
SIDERÚRGICA NACIONAL SIDENAL S.A. is located in the industrial park of Sogamoso (Department of Boyacá). This siderurgic annually process 250 000 tons of steel, manufacturing products for construction, such as bars, profiles, rebars, and meshes. SIDENAL is a semi-integrated siderurgic that uses scrap as raw material, which is fused in an electric arc furnace.
The company develops innovative projects aimed at improving competitiveness through energy efficiency and production increase. For this reason, the company signed an agreement with the Universidad Pedagógica y Tecnológica de Colombia (UPTC) through the Instituto de Investigación e Innovación en Ciencia y Tecnología de los Materiales (INCITEMA), and hired the services of SPIN OFF ENERMA at the Siegen University (Germany) to model processes and analyze products. The project was conducted between 2012 and 2015, and was funded by COLCIENCIAS (invitation 548 of 2012), which gives tax benefits for investing in Sciences, Technology and Innovation.
Before loading up the scrap to the electric arc furnace, a 5000 HP METSO shredder with approximate capacity of 800 t/day was used to reduce the scrap size. Shredding produces 11 % of waste that is composed of 12 % of non-magnetic metal (stainless steel, magnesium alloy, brass, zamak, aluminum alloy, cupronickel, cupper allow, and zinc alloy), 12 % of materials with organic compounds (elastomer, foam, plastic, fiber, rubber, and biomass), and 77 % of inert material (dirt, stone, and glass). 1400 t of waste are produced monthly, which motivated the study to recover materials, and assess their energetic value (Fig. 1 and 2).
This research aims to analyze the shredded scrap waste energetic transformation, and the material recovery through innovation: design, implementation, stabilization, and product analysis of a pilot plant of material separation and classification, and thermic processing of organic compounds to produce synthetic fuels.
II. State of the art
In order to produce scrap, reduction of its size is necessary, for which, usually, potent shredders and material separation and classification processes are used. Manufacturing plants that receive automobiles to be shredded have named the waste that is left after the ferrous material is separated as Auto Shredder Residues (ASR). Although shredded vehicles are only a fraction of the total scrap received by SIDENAL S.A., waste composition is highly similar to that reported in several studies on ASR treatment [2]. Other research groups have determined that the most suitable ASR treatment is the pyrolysis process due to its capability to recover materials, and transform the organic fraction into useful products for the siderurgic. In Colombia, projects regarding the ASR and its treatment by pyrolysis to produce fuels from solid organic residues are still lacking.
Pyrolysis plant suppliers recommend to begin with a pilot plant to scale to determine the design and development parameters of the definitive plant, as well as to validate the material's behavior, adoption, and production capability to recover fuels and other inorganic materials as indicated by Dr. Roy for the Canadian company PYROVAC [3], and for the German company SICON GmnH [4]. Additionally, Kubik [5] concluded that more applications of waste transformed by pyrolysis (according to the plant technology type), particularly in the cogeneration field, are expected. Finally, parametrization of the pilot pyrolysis plant control variables for the treatment of shredded organic residues in the siderurgic is fundamental to guarantee the stability and homogeneity of the obtained fuels [6].
III. Fundamental concepts of pyrolysis and metal separation by Eddy parasitic currents
Pyrolysis is defined as a thermic degradation in absence of oxygen that transforms raw material into several reactive intermediates products: solid (carbon), liquid (high-molecular-weight compounds that condense when cooled off), and gas (low-molecular-weight gases). The pyrolysis process is complex because of the many factors that must be considered, for instance, raw material composition, and experimental conditions.
In general, two possible steps in any pyrolysis process are accepted: i) primary pyrolysis, which involves devolatilization of the material when different reaction zones, corresponding to the thermal decomposition of the main components, may appear; and ii) secondary pyrolysis, which involves secondary decomposition with reactions in a solid matrix, as well as secondary reactions between volatile compounds (homogeneous reactions), or between volatile compounds and carbon residues (heterogeneous reactions). The first stage mainly implies dehydration, dehydrogenization, decarboxylation, or decarbonylation reactions. The second stage includes processes like cracking (thermic or catalytic), where heavy compounds get broken and transformed into gases or coal dust. These heavy compounds may be transformed into gases such as CO, CO2, CH4 and H2 as a result of reactions with reducing agents such as partial oxidation, or condensation and polymerization.
Another innovation of this project was the acquisition of an Eddy current (also called Foucault current) separator, which generates parasitic currents in nonmagnetic metals that, in turns, create a magnetic field that facilites current separation as described below.
Under certain assumptions (uniform material, uniform magnetic field, no skin effect, etc.), power loss, due to Foucault currents per unit of mass, of a thin film or wire may be calculated using equation (1)[7].
Where, P is the power loss per unit of mass (W/kg), Bp is the peak magnetic field (T), D is either the film thickness or the wire diameter (m), F is the frequency (Hz), K is a constant equal to 1 for a thin film, and equal to 2 for a thin wire, P is the material resistivity (Ω m), and D is the material density (kg/m3).
Parasitic currents induced in the non-magnetic metal fractions facilitate the separation of the metals from the scrap waste current, thus allowing the scrap waste recovery, and the cleaning of the material that will be processed in the pyrolysis plant.
IV. Content of this study
A. Scrap waste characterization
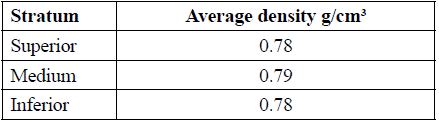
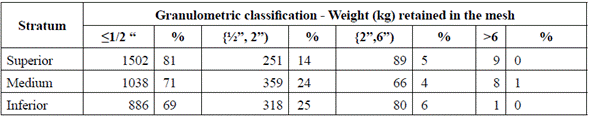
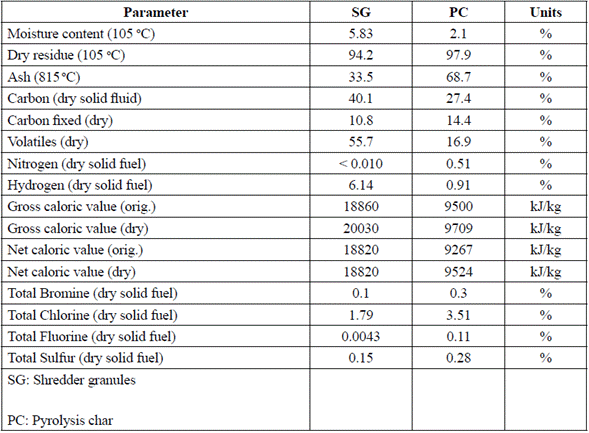
Initially, sampling of the 76 000 tons of accumulated waste was designed and applied according to the ISO 2859-1:1999 standard. We selected 8 georeferenced locations, and stratified the inferior, medium, and superior levels of residue density and size for each one of them, based on the EPA practice recommendations [8]. The characterization of the scrap waste is shown in Tables 1 through 3.
Despite the height of the waste pile, it is possible to observe that the material has uniform density, without any compaction effect, which ease the material analysis. Additionally, to characterize the particle size of the materials composing the pile of waste, three meshes were employed: ½", 2'', and 6''. A higher proportion of particle sizes below ½", and a lesser proportion of sizes above 6" were observed (Table 2).
Finally, the energetic potential of the carbonaceous material in the pile of waste showed a calorific value superior to 1210 kcal/kg, which indicates the utility to process it as fuel (Table 3).
B. Pyrolysis process
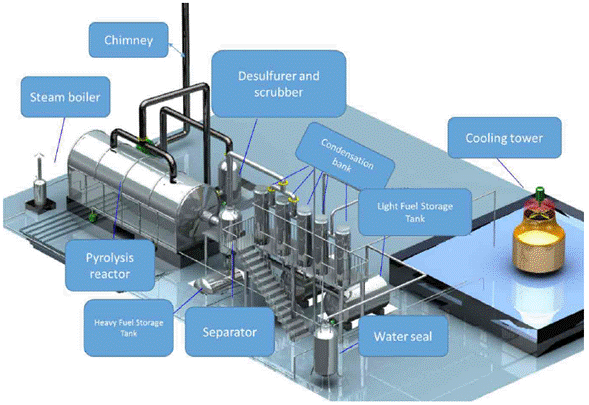
Based on the characterization of the materials composing the pile of waste, the material separation equipment (screening and Eddy currents) of the pilot plant was designed, selected, and installed. Once the materials were separated and classified, the organic fraction was processed in a pyrolysis plant that thermally depolymerized the carbonaceous material in the absence of oxygen, and at a pressure higher to the atmospheric one. The pilot plant is composed of a rotating horizontal reactor, a solid-liquid separation system, a vertical capacitor bank to separate liquidgas phases, an anti-explosion system with water traps, a gas burner/scrubber, a boiler to produce vapor, a cooling tower, storage ducts and tanks, a digital instrumentation and control board, a dual fuel system (gas-liquid), and an alarm system (Fig. 3 and 4).
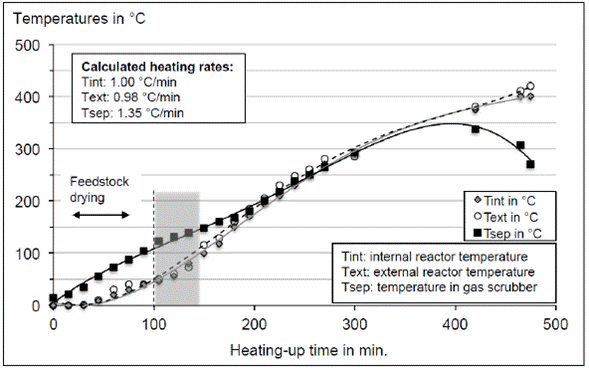
For plant balance, we conducted simulations and thermodynamic analyses of mass, energy, and gases kinetics, with the goal of estimating the ideal operation conditions to achieve a maximum efficiency in the transformation into synthetic fuels (light and heavy fuels, gas, and pyrolytic coke) [10]. The optimal operation curve for time and temperature, where the lowest fuel production was achieved (Fig. 5), allows to evaluate the temperature gradient, and the correlation between the pyrolysis reactor external and internal temperatures, as well as the gas temperature inside the scrubber.
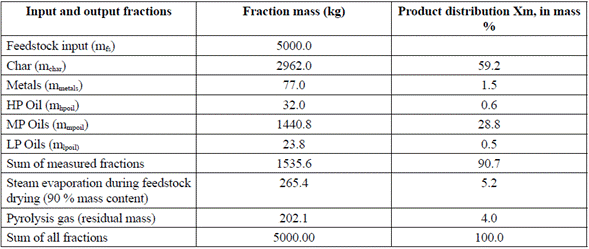
The results of the plant mass balance showed that most of the transformed material in the pyrolysis reactor is pyrolytic char, followed by medium molecular weight synthetic oil (Table 4).
C. Products of the scrap waste pyrolysis
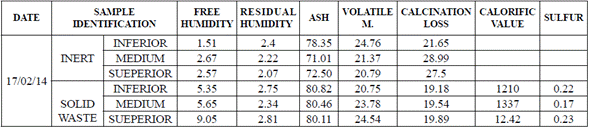
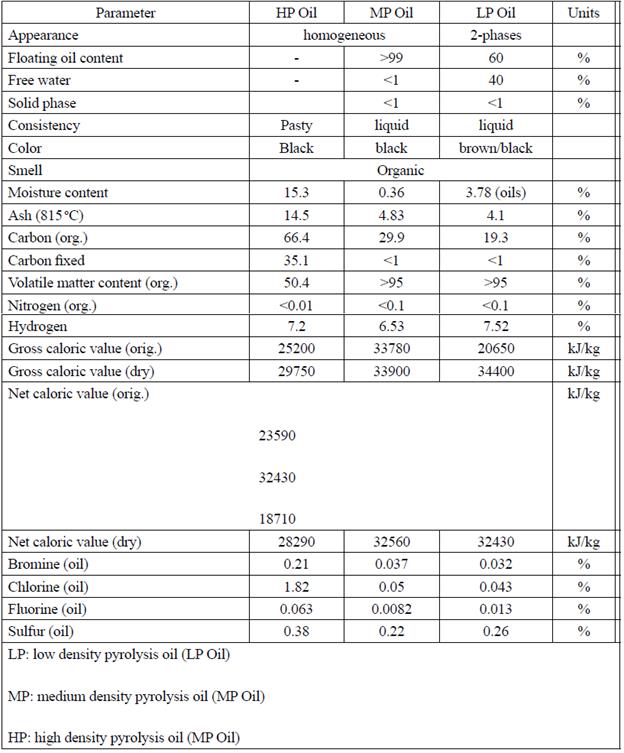
The characterization of the synthetic fuels obtained at the scrap waste pilot plant showed that the pyrolytic char has an important energetic potential, and a high fraction of ash that anticipates the necessity of an additional separation process to concentrate the carbon fraction indicated by the analysis (Table 5 and 6).
The analysis of the synthetic oil fuels produced in the pyrolysis process indicated that the medium molecular weight oil was not only the most produced, but also the one with the best energetic behavior (Table 6). Additionally, it is noteworthy the presence of compounds such as bromine, chlorine, fluorine, and sulfur (Table 6).
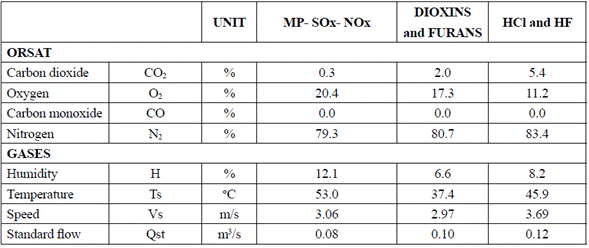
Emission analysis (Table 7 and 8) was conducted to verify that emissions were in compliance with the environmental normativity, particularly with resolution 909 of 2008.
V. Results
This study demonstrated that innovative technology of material recovery and energetic transformation into synthetic fuels from scrap waste, by means of pyrolysis allowed in SIDENAL:
To monthly recover more than 960 tons of soil to be potentially used as an agricultural substrate or fertilizer.
To monthly recover more than 210 tons of metal to recycle.
To transform every month 330 tons of organic compounds into synthetic industrial fuels: 30 % oil, 60 % char, and 10 % gas.
The pilot plant allowed to develop the innovative technology to process shredded solid waste to move the project forward to a second phase, which initiated thanks to the benefits granted by the Agencia Nacional de Licencias Ambientales (ANLA). The investments will take place throughout the upcoming years. In phase II, stored and fresh waste will be processed, with a capacity of 200 t/day.
The technology that will be used is highly reliable, and comes from German companies that comply with the strict European and North American environmental regulations for this kind of activities.
This project at this scale is a real referent to promote close cycle industries, and to recover materials from syderurgics and sanitary landfills.
VI. Conclusions
The synthetic fuel characterizations suggest to develop the following uses in SIDENAL:
Fuel oil: Steel billet furnace. Mixed with the current fuel, and adjusting a filter system, 30 % of the fuel might be substituted with only one reactor of 10 tons.
Gas: pyrolysis reactor realignment, adjusting burners, and a pressure regulation system for the supply system. Moreover, syngas might be used in a cogeneration system (electric-thermal), utilizing the heat residue to dry the material that entries the reactor.
Coke or char: briquette to be used in cement industry, mixing it with fluff residues, for which an additional separation system is required.
Inert material: mixed with whitewashing material that comes from white slag could be useful for soil remediation.
The study had the following conditions:
The characterized material was sampled in the pile of waste by size and shape.
Additional control variables such as flow, pressure, and gas analysis in real time were reported and monitored.
Meshes of different sizes were used to sieve the residues in thinner fractions.