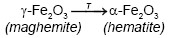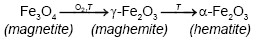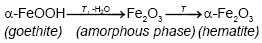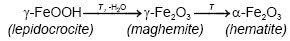I. Introduction
In recent years, the recycling of solid waste rich in iron to obtain iron oxides has become a major research topic. For example, residues such as mill scale that is considered a waste in the steel rolling industry, can be used as raw material to synthesize pigments of red iron oxide (hematite), due to its stable chemical composition, high content of iron and low level of impurities [1]. This is possible due to the interconversions that suffer the different phases of precursor iron oxides after being subjected to thermal treatment under oxidizing conditions (calcination).
The transformations that involve physicochemical modification are: i) dehydration (loss of H2O), (ii) dehydroxylation (loss of OH), and (iii) structural transformation processes (topotactics) [2]. The final stage in most methods of iron preparation (III) oxides includes a thermal transformation of the iron-containing material. In some cases, thermal processes can be used as direct routes for the synthesis of iron oxide particles, such as hematite, which has a good physicochemical stability. The main advantage of direct thermal methods is the simplicity of the preparation, but its application depends on a large extent on the nature of the initial iron-containing sample [3].
Various types of steel waste (mainly mill scale) and other similar residues (such as those from mining) have been used, since they contain precursor iron oxides that allow total conversion to hematite, after direct thermal treatment of these ones (a different temperatures and times of calcination). These residues mainly constituted by iron in a proportion not much higher than 60% (expressed as Fe2O3) and compounds of Si, Ca, Al, Mn, etc. [4], [5], [6], [7], [8]. For example, Sikalidis et al. [9] obtained hematite by calcining at various temperatures (275, 500, 700 and 850 °C) for 1 h, a powdery residue derived from the chemical steel pickling lines, which was mainly composed by iron (48 %Fe2O3) corresponding to several iron oxyhydroxides, mostly lepidocrocite (γ-FeOOH) and goethite (α-FeOOH).
In accordance with the above, it is important to know that the waste generated during the manufacture of the steel, such as the mill scale, can be of various types and, therefore, exhibit different properties, since as indicated by Ovčačíková et al. [10], it can occur the creation and reduction of iron oxides with higher valencies according to the initial composition of the different iron oxides. Furthermore, as it is explained by Legodi and de Waal [11], this mill scale can also be accompanied by the precipitation of a mixture of other corrosion products, e.g. FeOOH. Therefore, according to Zitrou et al. [12], it can be said that layers of iron oxides formed on carbon steel surfaces, such as in the reinforcement bar steel, initially contain wustite (FeO), magnetite (Fe3O4) and hematite (Fe2O3), as products of hot rolling, and after exposure to the atmosphere, the main product formed is lepidocrocite (γ-FeOOH), in addition to akaganeite (β-FeOOH) and goethite (α-FeOOH). Coincidentally, some of these iron oxides occupy a preferential place as anticorrosive pigments; especially hematites or red iron oxides (α-Fe2O3), as is points out by Cuesta [13], are the pigments most commonly used to make anticorrosive paints.
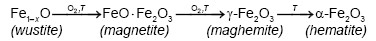
On the other hand, Emira et al. [14] demonstrated the effectiveness of the use of a waste generated in the manufacture of steel, after of washing with hot water and grinding, to finally it be used as a pigment in an anticorrosive paint, which was successful thanks to the anticorrosive action offered mainly by the content of zinc ferrite (ZnFe2O4) and ZnO of the processed waste. In the same sense, it should also be noted that certain specific corrosion products of steel, such as maghemite, magnetite, lepidocrocite, wustite and goethite, are transformed into hematite according to equations (1), (2), (3), (4) and (5), after to be subjected to thermal treatment [15], [16], [17], [18], due to the well-defined structural relationships between these oxides and iron oxyhydroxides [19].
Therefore, the objective of this research is to obtain an anticorrosive pigment with a high content of hematite and a low content of impurities, by calcination of precursor phases present in a residue coming from the surface oxide scale of steel reinforcing bars (steel rebars).
II. Methodology
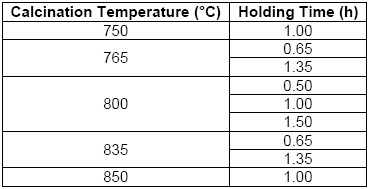
As an industrial waste, were collected 3.77 kg from the manual cleaning process of steel reinforcing bars, after of passed the as-received sample through a 200 mesh sieve (with aperture of 74 microns). After this, the residue was dried at 60 °C for 24 hours in a convection oven and pulverized in a bar mill with a 40% filling, at a speed of 32 rpm for a period of seven hours per kilogram. Finally, this processed waste was labeled as “PR”. For the thermal transformation of the PR, a Nabertherm brand muffle furnace (type LE 14/11) was used to calcinate two samples of 5 g of residue in porcelain crucibles. The specific conditions of the thermal treatment implied a calcination temperature with their respective holding times, which can be seen in Table 1 [4], [5], [6], [7], [8], [9]. The heating rate used was approximately 24 °C/min. At the end of the heating stage, the samples were first cooled slowly to 300 °C (closed oven) and then rapidly to 100 °C (open oven) [15], [16], [17], [18], [19]. Finally, the samples were taken and labeled considering the sample number (S) and the respective calcining condition where the pigment (P) was obtained.
Regarding the identification and quantification of the elements that constitute the powder residue (PR), the X-ray fluorescence (XRF) technique was used, using a wavelength dispersion X-ray fluorescence spectrometer (WDXRF) brand Philips model MagixPro PW-2440, with rhodium power and maximum power of 4 KW. Otherwise, to determine the crystalline phases of the residue and of the pigments obtained at different calcination conditions, the powder X-ray diffraction (PXRD) technique was used by means of an EMPYREAN model 2012 diffractometer with Co-Kα radiation (λ=1.7890100 Å), operated at 40 kV, 40 mA, in an angular range of 2θ=5-80° with a step of 0.026° [20], [21].
The qualitative identification of the main crystalline phases of the waste was restricted by associating the elemental composition obtained by XRF with the possible theoretical phases that may be in the residual material, discarding the components with a concentration greater than 0.9%. Finally, this procedure for the residue was analyzed in the angular range of 2θ = 20-80° [20], [22], through the use of the X'Pert HighScore Plus® v3.0 program from PANalytical® and the Database from the International Center Structural Database (ICSD 2012), after it were chosen the suggested theoretical patterns that had the greater coincidence with the PR diffractogram (experimental pattern generated with the acquired data by PXRD) [23].
III. Results and Discussion
The results of the semi-quantitative analysis of XRF determined that the processed waste (PR) was mainly composed by iron (87.92 %Fe2O3), silicon (6.13 %SiO2), calcium (1.88 %CaO) and aluminum (1.30 %Al2O3). Small amounts of other elements were also observed: Mn, S, Na, P, Mg, K, Cr, Ti and Zn. The elements identified in this residue coincide with the components reported in similar residues with high iron content [4], [22].
Therefore, only the possible identification of crystalline phases of the main elements obtained (Fe, Si, Ca and Al) was taken into account, which could also be associated with other elements that could not be detected by XRF (such as O and H), in according to said by Jaramillo [23] describes in her protocol for the identification of crystalline phases. After the execution of the X'Pert HighScore Plus® program, the crystalline phases chosen from the list of candidates (theoretical patterns suggested and ordered by the program according to their coincidence with the experimental pattern) were associated to the PR diffractogram.
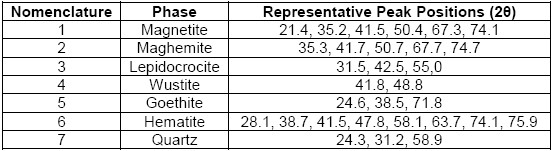
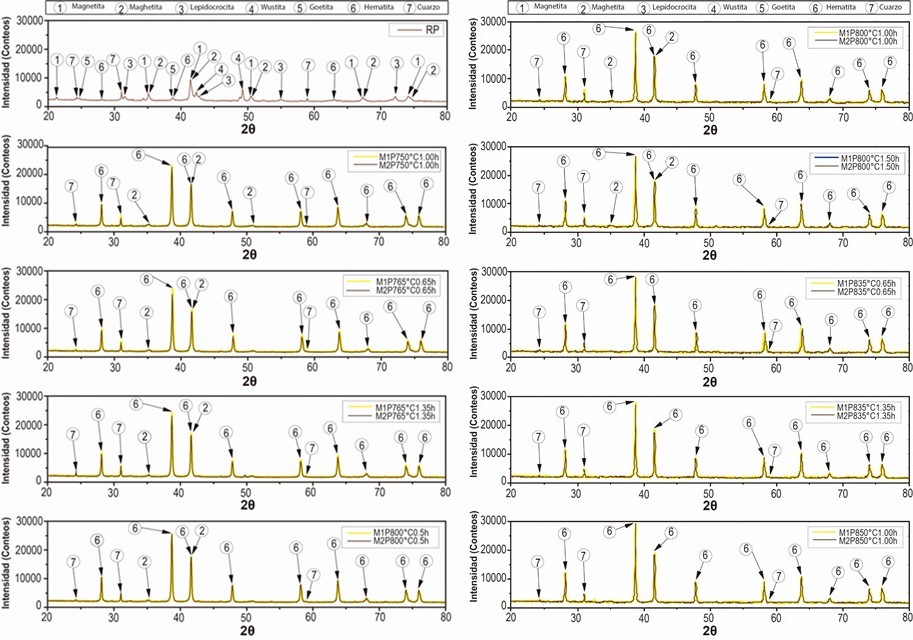
From the above, there was a good correspondence that mostly related iron oxide phases (magnetite, maghemite, lepidocrocite, wustite, goethite and hematite) to the experimental pattern of PR, which clearly agree with the high iron content reported by the results of XRF and with the phases of iron oxides that are generally found in steel corrosion products [19], [24]. Moreover, the presence of quartz was also in accordance with the results of XRF and to the usual presence of this phase that is reported as impurity in similar industrial wastes [5], [7]. Therefore, they were identified the seven chosen phases (numbered from 1 to 7 in the Table 2) in the PR diffractogram (Figure 1), after considering the approximate 2θ position of the most representative peaks of their associated theoretical patterns, which are also summarized in Table 2.
Although most of the iron oxides of the residue also coincide with all the phases that make up the carbon steel rod oxide scale reported by Zitrou et al. [12], and identified by XRD with copper source, the maghemite does not appear in their list of iron oxides, since they only identified magnetite, ruling out the existence of maghemite, which is also common to find it as a product of atmospheric corrosion of the steel, according to Alcántara et al. [25]
The explication of that situation was summarized by Mos et al. [20], since they demonstrated that a proper distinguishing of these phases by XRD is only possible with Co-Kα radiation (λ=1.7890100 Å) instead of Cu-Kα (λ=1.540552 Å), since the source of cobalt offers the resolution necessary to distinguish these phases or a mixture of them (γ-Fe2O3/Fe3O4) due to its greater depth of penetration in iron-rich samples, as is also points out by Whitfield [21]. According to the above, the identification of these phases would be incomplete in steel residues that have been widely described with diffractograms obtained with a copper source [1], [4], [5], [6], [8], [9], [10], [11].
From Figure 1 is considerable the variation of the precursor phases (1, 2, 3, 4 and 5) in hematite (6), before and after the thermal treatment of the processed residue. The diffractograms of the two replicas of the obtained pigments show a very similar behavior in the different treatments where is observed a clear enrichment of the hematite from a calcination temperature of 750 °C. The comparison of the diffractograms of the PR and of the pigments obtained, evidences that the increase in the calcination temperature drastically decreases the original maghemite content due to its conversion into hematite, since it begins to transform from approximately 370 °C, as explain Cornell and Swchertmann [2]. For example, the diffractograms of S1P800°C1.00h and S2P800°C1.00h, show a reduction in the contribution of the maghemite and a clear increase in the intensity of representative peaks of the hematite.
It can also be observed that the maghemite practically disappears from a calcination condition of 835 °C and 0.65 h. After calcining the residue (PR) at 850 °C for 1 h, the highest peak of hematite was obtained, which is in agreement with that obtained by Sikalidis et al. [9] It should be noted that other major phases such as magnetite, wustite and lepidocrocite completely disappear in all the calcined samples, since these phases are completely transformed at calcination temperatures between 600-800 °C [8], [9]. Apart from the precursor phases, it can be seen that the thermal treatment used did not affect the quartz contribution of the residue diffractogram (PR), which can be verified by monitoring the height of the representative peaks of this crystalline phase. This behavior follows the same tendency of the residue of mining origin used by Aguaiza and Aldás [7].
Ultimately, when confronting all the diffractograms with each other it was observed that as the calcination temperature was increased (e.g. when comparing the P750°C1.00h and P850°C1.00h), a considerable growth of the most representative hematite peak was distinguished, while when the holding time was increased (e.g., when comparing P765°C0.65h and P765°C1.35h), the growth of hematite was not so evident.
IV. Conclusions
The results of the qualitative analysis of diffractograms confirmed the direct synthesis of hematite by thermal treatment of a residue mainly composed by corrosion products from carbon steel rebar. Likewise, it was evidenced that the best calcination condition to synthesize hematite was a temperature of 850 °C and a holding time of 1.00 h, since a pigment with a high content of hematite was obtained, which practically only had quartz impurities, thus being suitable for application in anticorrosive paints [25].
In addition, according to the diffractograms acquired by PXRD with cobalt radiation afterwards of the different thermal treatments, the formation of hematite at temperatures above 750 °C was appreciated, which indicates that the calcination promoted the conversion of precursor oxides into hematite. Thus, it was shown that the calcination temperature exerts a strong influence on the interconversions of these crystalline phases, since, as is outlined by Olmedo [19], these structural transformations between iron oxides and oxyhydroxides occur at high temperatures under oxidizing conditions.
Author’s contributions
The contribution in the work by the authors was made as follows: José A. Pérez proposed the pretreatment of the recovered residue, Camilo Gnecco and María A. Colpas identified the crystalline phases of the diffractograms obtained, Óscar F. Higuera analyzed the influence of the heat treatment on the waste and Gabriel A. Jiménez suggested that the pigments obtained can be implemented in an anticorrosive paint.