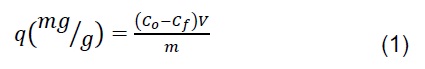I. Introduction
Surface and underground water pollution by toxic heavy metals is the product of dumping industrial effluents [1]. Chromium is a heavy metal dumped at high concentrations to the environment, which is caused by industrial activities such as the fabrication of fertilizers, textiles, photography, pigments, tannery, electroplating, electronic manufacture, among others [2, 3]. After coming in contact with water, this metal is found in two oxidation states: Cr(III) and Cr(VI). Usually, Cr(VI) is found as chromate ions (CrO4 2-) or dichromate (Cr2O7 2), which enter easily in contact with biologic barriers, presenting carcinogenic and mutagenic behaviors, besides, Cr(VI) is 500 more times toxic and moves better than Cr(III) [5, 6]. Although Cr(III) is not toxic, when is encountered in aqueous solutions may become toxic by oxi-reduction to Cr(VI) [7].
Several methods have been reported for the removal of this metal due to the high resistance to degradation and biomagnification, such as: precipitation, oxidation, reduction, ionic exchange filtration, electrochemistry treatment, membrane technology, neutralization, electrocoagulation, inverse osmosis, catalytic oxidation, bioremediation, electrokinetic and evaporation [8,9,10]. However, implementation of these technologies implies high consumption of energy, operational costs, chemical additives, sludge accumulation and low efficiency at low concentrations. In this context, biosorption is an alternative against the elimination of heavy metals from industrial effluents with the aim to achieve high removal percentages, since the adsorbents from agroindustrial wastes present excellent properties for maintenance, low cost, easy processing, good reusability and wide availability [2, 9]. Among the materials widely studied with excellent results for the removal of Cr(VI) in aqueous solutions, are found: peels of lime [7], rice and lychees [4, 5], orange [11], apple [12], nut [13, 14], lemon [15] plantain [10, 16], among others [3, 17, 18]. Hence, this study compares the Cr(VI) adsorption capacity using orange peels and corncob as bioadsorbents, performing a batch system. Biomass was chemically modified with citric acid (corncob) and calcium chloride (orange peel) with the aim to determine the effects of the functional groups to the adsorption capacity of Cr(VI) ions.
II. Materials and methods
A. Preparation and characterization of the bioadsorbents
Orange peels and corncob were washed with distilled water and dried at 70 °C for 24 h, and then the particle size was reduced by using a rolling mill and classified with sieving through 1, 0.5 and 0.555 mm meshes. Biomaterials where characterized through elemental analysis using AOAC 949.14 method to determine the percentage of carbon, nitrogen and hydrogen. The content of lignin was determined by photocolorimetry, and content of pectin and hemicellulose by acid digestion, cellulose and ashes by basic digestion using thermogravimetry. Fourier transformed infrared spectroscopy (FTIR) was performed to determine the functional groups that intervene in the adsorption process by using a NICOLET 6700 with an absorbance range between 4000 and 500 cm-1.
B. Modification of biomaterials
Corncob was modified chemically by mixing 13.5 g of biomass with 33.75 mL of a 0.6 M citric acid solution at 60 °C for 2 h [14]. Orange peels crosslinking was achieved by putting in contact 20 g of biomass with 500 mL of 0.2 calcium chloride solution at 200 rpm for 24 h [19]. After the treatment with citric acid and calcium chloride, the products were washed several times with distilled water to remove the excess of reactants, and then were filtered and dried at 60 °C for 24 h [1].
C. Adsorption experiments using a batch system
A 100 ppm solution of potassium dichromate (K2Cr2O7) was used for the adsorption experiments, in which 0.5 g of bioadsorbent was added to 100 mL of the solution at different pH (2, 3, 4 and 6) and the particle size defined previously. The solution was placed into an incubator shaker IN-666 at 150 rpm and room temperature for 2h. Then, 0.5 N solutions of HCl and NaOH were used to adjust pH. Moreover, Cr(VI) concentration was measured following the standard method ASTM D 1687-02, in which 1,5-Diphenylcarbazide was used to be read at 540 nm in an UV-Vis spectrophotometer Shimadzu UV 17000. Quantity of adsorbed Cr(VI) in the biomass was determined through the Equation (1).
Where Co and Cf (mg/L) are the initial and final concentration of Cr(VI), respectively, V (L) is the solution volume and m (g) is the bioadsorbent mass used in the experiments.
D. Adsorption kinetics and istoherms
Adsorption kinetic was studied to determine the equilibrium time in which the biologic material reaches the highest adsorption of Cr(VI). Experiments were performed putting in contact 0.5 g of biomass with 100 mL of Cr(VI) solution at 100 ppm, using the best conditions for pH and particle size as determined previously. Aliquots of 5 mL were taken each certain time between 10 and 330 minutes [10].
Adsorption isotherms were performed to describe the equilibrium of the solute between the solid and liquid phases. Experiments consisted in using different initial concentrations of Cr(VI) (25, 50, 75 and 100 ppm), taking 0.5 g of biomass with the best condition stablished previously. The contact time between the biomass and the solution depended on the equilibrium time achieved in the adsorption kinetics [11].
III. R esults
A. Characterization of biomaterials
Elemental composition of the biomass is shown in Table 1. Carbon content was determined to be 44.4% for the orange peels and 40.0% for the corncob. Results provided the presence of hydrogen, nitrogen and other biopolymers which are usual in this type of biomass, showing similar information as is reported in the literature by other authors [13, 20].
Table 1 Elemental composition of Orange peels and corncob
| Parameters | Orange peels | Corncob | Analytical method |
| Carbon | 44.40 % | 40.00 % | AOAC 949.14 |
| Hydrogen | 6.21 % | 3.28 % | AOAC 949.14 |
| Nitrogen | 0.81 % | 0.45 % | AOAC 984.13 |
| Pectin | 18.10 % | 4.88 % | Acid digestion – thermogravimetric |
| Lignin | 7.14 % | 17.11 % | Photocolorimetry |
| Cellulose | 14.28 % | 19.90 % | Digestion - thermogravimetry |
| Hemicellulose | 7.02 % | 7.00 % | Acid digestion – thermogravimetric |
| Ashes | 2.04 % | 4.23 % | Digestion - thermogravimetry |
| Minerals and others | 6.39 | 3.15 % | EAA graphite furnace |
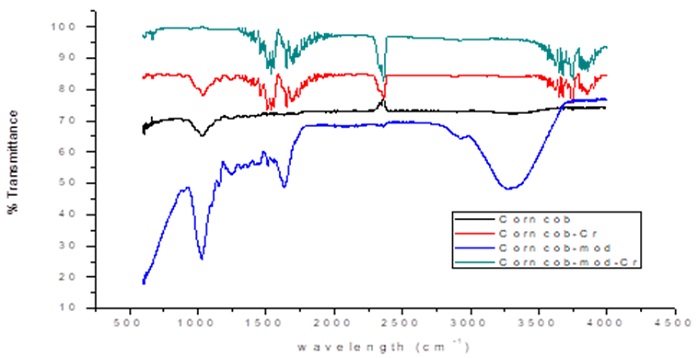
Biomass was analyzed through FTIR with the aim to determine the chemical structure of the bioadsorbent materials, and those functional groups with affinity towards heavy metal ions such as Cr(VI). In Figure 1, the infrared spectra show the results for the unmodified and modified corncob after the adsorption process.
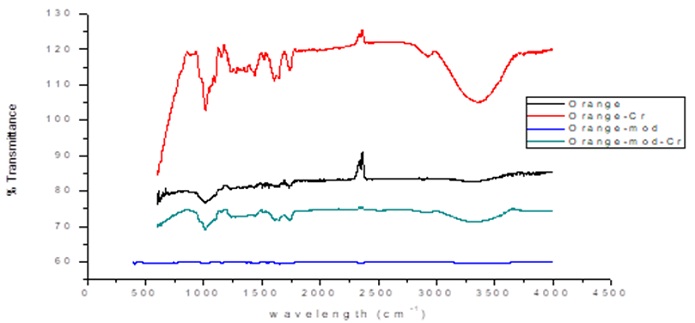
In Figure 2, FTIR spectra shows the results for the unmodified and modified orange peels, before and after the adsorption process.
B. Effect of pH and particle size
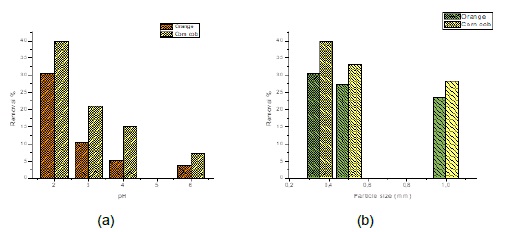
Adsorption is influenced by the pH of the solution affecting the superficial charge of the adsorbent, the ionization grade and the specie of the sorbate [16]. In Figure 3a is evidenced the effect of pH in the removal percentage of Cr(VI), observing an increasing in the adsorption percentage of the biomaterial as pH decreases in the solution. Removal percentages of 39.8% and 30.6% were achieved for corncob and orange peels at pH 2, respectively.
C. Effect of contact time
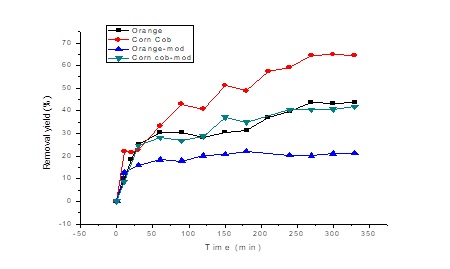
The study of contact time provides information related to the practical uses of the sorbent, as well as the velocity of the sorption process [25]. The effect of contact time during the removal of Cr(VI) using orange peels and corncob before and after modification, is shown in Figure 4. From the curve is evidenced that the elimination of Cr(VI) increased and the equilibrium was achieved at 270 min.
D. Kinetic adsorption
Experimental data obtained was adjusted to the kinetic models of Pseudo-first order [23], Pseudo-second order [26] and Elovich [13]. In Figure 5a is shown the curves for the unmodified and modified orange peels after the adsorption of Cr(VI), and in Figure 5b is presented the curve for the case of corncob.
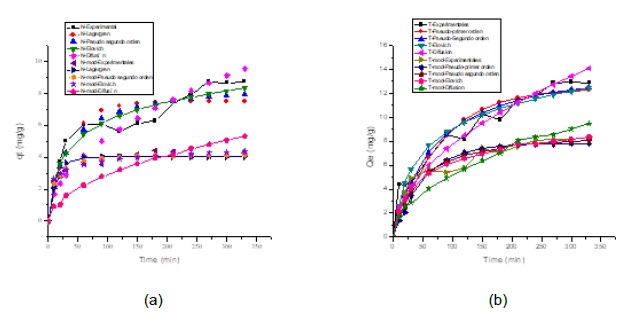
Fig. 5. Experimental data adjusted using (a) unmodified and modified orange peels and (b) unmodified and modified corncob
The parameters valued for the adjustment of the models, the sum of the error (SS) and the correlation coefficient (R2) are shown in Table 2.
Table 2 Parameters for the adjustment of the adsorption of Cr(VI) using unmodified and modified orange peels and corncob.
| Model | Parameter | Orange | Modified orange | Corncob | Modified corncob |
| Pseudo-first order | qe1 | 7.479 | 4.022 | 12.483 | 7.809 |
| k1 | 0.028 | 0.079 | 0.0127 | 0.019 | |
| SS | 11.762 | 0.872 | 17.478 | 6.285 | |
| R2 | 0.930 | 0.974 | 0.969 | 0.961 | |
| Pseudo-second order | k2 | 0.004 | 0.029 | 0.001 | 0.003 |
| qe2 | 8.664 | 4.268 | 15.052 | 9.000 | |
| SS | 7.192 | 0.331 | 11.841 | 3.560 | |
| R2 | 0.957 | 0.989 | 0.975 | 0.977 | |
| Elovich | β | 0.592 | 2.062 | 0.353 | 0.559 |
| α | 0.699 | 11.581 | 0.695 | 0.578 | |
| SS | 4.887 | 0.351 | 10.545 | 2.471 | |
| R2 | 0.971 | 0.989 | 0.974 | 0.984 |
E. Adsorption equilibrium
The adjustment of the experimental adsorption equilibrium data of Cr(VI) ions using modified and unmodified orange peel and corncob are shown in Figure 6. The Langmuir and Freundlich isothermal models were evaluated by nonlinear regression in order to establish the driving forces governing the interaction between adsorbate and adsorbent and the adjustment parameters are shown in Table 3.
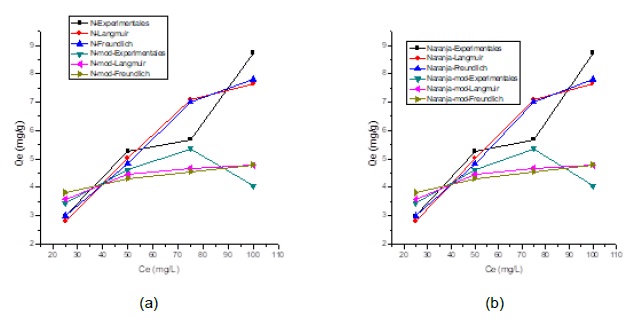
Fig. 6. Adjustment to adsorption isothermal models using: (a) orange before and after modification with CaCl2 and (b) corncob before and after modification.
Table 3 Parameters of Freundlich and Langmuir for Cr(VI) adsorption.
| Model | Parameters | Orange | Modified Orange | Corncob | Modified Corncob |
| Langmuir | qmax | 12.292 | 4.952 | 14.813 | 208.925 |
| B | 0.029 | 0.334 | 0.127 | 0.001 | |
| SS | 3.239 | 1.075 | 1.645 | 7.057 | |
| R2 | 0.899 | 0.685 | 0.979 | 0.830 | |
| Freundlich | Kf | 0.833 | 3.117 | 3.035 | 0.161 |
| 1/n | 0.555 | 0.098 | 0.399 | 0.940 | |
| N | 1.801 | 10.204 | 2.506 | 1.064 | |
| SS | 2.845 | 1.447 | 0.767 | 6.974 | |
| R2 | 0.911 | 0.534 | 0.990 | 0.828 |
IV. Discussion
The spectra of unmodified biomasses before the adsorption process show peaks at wavelengths of 3300 cm-1, characteristic of hydroxyl group (-OH) stretching which according to the literature are in the range of 3400-3000 cm-1 [21]. In addition, peaks around 1732 cm-1, 1615-1684 cm-1, 1558 cm-1, and 1235 cm-1 were also detected, corresponding to the stretching of the carbonyl groups (C=O), double bonds between carbons (C=C), bonds between carbon atoms and hydrogen (C-H), and bonds between carbons and oxygen (C-O), respectively [20]. These functional chemical groups are characteristic of the chemical structures of the carboxylic, aromatic, alcohol, methyl and methoxy groups present in the pectin, hemicellulose and lignin components of the studied biomasses [3].
The corncob and orange peel after modification with citric acid and calcium chloride, respectively, show a widening in the peaks between 3000 to 3500 cm-1 corresponding to the vibration of the hydroxyl groups; it is also observed that the peak about 1300 cm-1 corresponding to the bonds between carbon and oxygen. In the corncob modified with citric acid the creation was presented in 1680 corresponding to the presence of carboxylic acids, confirming that the modification was effective. On the other hand, in the spectrum of the orange peel after the modification with calcium chloride a peak between 540 and 700 cm-1 appeared corresponding to the presence of chloroalkanes in the structure of the material, thus confirming the effectiveness of cross-linking with CaCl2 [19]. The spectra of the biomaterials after the adsorption process show that most of the peaks present a considerable increase in the intensity and width of the bands, due to a slight variation in the frequency of adsorption, which could be attributed to the union of the Cr (VI) ions with the different chemical groups present in the biosorbents [15].
The results obtained can be attributed to the fact that at lower pH, the surface of the biosorbent is highly protoned by the H+ ions, so there is a greater attraction between these and the chromium ions; in the pH range 2-6, the predominant form is Cr2O7 2- and HCrO4 -, this last one being the predominant species at acid pHs [6]. The diminution of electrostatic attraction causes the decrease of adsorption as the pH of the solution increases, which is caused by the competition between the anionic species of chromium (HCrO4 - and CrO2- 4) and the OH- ions in the solution for adsorption in active spots which leads to the generation of repulsive forces between the Cr (VI) ions and the biosorbent, which inhibits adsorption and consequently decreases adsorption of Cr (VI), being the electrostatic interaction a predominant mechanism in the elimination of Cr (VI) using orange peel and corncob [5]. A similar behavior has also been reported previously using rice husks [5], walnut shells [13], and other materials of vegetal origin [3, 22].
The adsorption takes place mainly inside the particles, on the walls of the pores at specific points. The amount of adsorbate (solute) that can be adsorbed is directly proportional to the volume of the pores, and this volume is directly proportional to the external area. In Figure 3b, the particle size is related to the percentage of adsorption of Cr (VI) using orange peel and corncob. The highest removal percentage obtained for both biomasses at pH 2.0 was achieved using the smallest particle size evaluated, that is 0.35 mm (~31% for orange peels and ~40% for corncob). This behavior may indicate the possibility that adsorption of metal ions may also occur inside biomass particles through the porosities of these biomaterials. In addition, a small particle has a larger surface area, increasing the number of pores per unit mass [24]. This means that as the size of the particles decreases, the number of active centers per unit increases and the adsorption capacity increases [15]. In the present study, corncob showed a higher percentage of Cr (VI) ion removal than the orange peel, at the pH conditions and particle size evaluated; this may be due to the higher content of cellulose and lignin in the structure of the material [22].
Figure 4 shows that it gives a fast elimination of Cr (VI) in the initial stages of the process using unmodified biomass, which may be due to the availability of the uncovered surface and the active sites on the adsorbent surface [22]. The results obtained from the different Cr (VI) adsorption tests carried out with the modified biomass, reflected a decrease in the adsorption potential of both biomass according to Figure 3. The highest removal percentage obtained by the corncob without modify was 64.5% after 270 min and modified it was 41.8% after 330 min; on the other hand, the unmodified orange peel obtained 43.7% removal and a modified 21.2%, after 330 min. This could occur due to the surface coating of the biomass instead of chemical modification [9, 25].
The maximum adsorption capacity for the orange peel and the unmodified corncob was determined with equation 1 and was obtained after 270 minutes after the start of the process, when equilibrium was reached. From the adjustment of the data obtained, the sum of errors (SS) and the correlation coefficient (R2) of Table 2, it was established that the Pseudo-Second order model describes the behavior of the adsorption process using the orange peel and the unmodified corncob, therefore it is established that the ion can be adsorbed by two active sites of the biomass and that the process occurs by chemisorption due to the formation of chemical bonds between adsorbent and adsorbate on the surface [12]. However, the Elovich model is also a good fit, so it can be said that the adsorption of Cr (VI) also takes place inside the pores of the bioadsorbent material particles and that their active sites are heterogeneous, so they exhibit different activation energies throughout the adsorption process [24]. The adsorption kinetics of Cr (VI) on orange peel modified with Calcium Chloride and corncob modified with citric acid, was adjusted by the Pseudo-Second order and Elovich models, this suggests that the speed limiting step of this adsorption system can be controlled by chemisorption that involves valence forces by sharing or exchanging electrons between sorbent and sorbate [18, 21, 24]. Similar conclusions were documented in the removal of Cr (VI) for ground biosorbents of ground tea and coffee [20], pineapple peels [26], and sweet lime peel [18].
From Figure 6, it is established that the isothermal model that describes in a better way the adsorption of Cr (VI) for the orange peel and the modified and unmodified corncob, considering values recorded for the statistical error (SS) and the coefficient regression (R2) (Table 3), is that of Freundlich. According to this model the surface of the biomass is heterogeneous, in addition during the adsorption process multilayers are formed on the surface of the biomaterials with an uneven distribution of heat and adsorption affinities on the heterogeneous surface, and that the active sites of Sorption are first occupied by strong bonds, and such force decreases as they are occupied by Cr (VI) ions [20, 26].
The value of the Freundlich constant n is in the range 1-10 which indicates that the chemical bonds formed between Cr (VI) and adsorbent are strong, and that the adsorption process is favorable. The adsorption intensity, given by the Freundlich parameter (n) is high compared to other works using adsorbent materials of plant origin for Cr (VI) [24, 26]. The Freundlich parameter (n) confirmed that the process can be classified as favorable for adsorption [10, 21].
V. Conclusions
The characterization of the biomaterials shows the presence of hydroxyl, carbonyl and carboxyl groups, belonging to cellulose and lignin, which are attributed to participation in the adsorption process. It was found that the best conditions for the removal of Cr (VI) using corncob and orange peel are pH 2 and particle size 0.355. The adsorption kinetics were adjusted by the Pseudo-Second order model, and the isotherms by the Freundlich model, which suggests that the process is controlled by chemical reaction and occurs in multilayers. The corncob has a Cr (VI) adsorption capacity greater than the orange peel under all the conditions tested in this study.