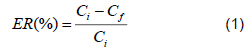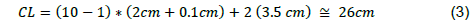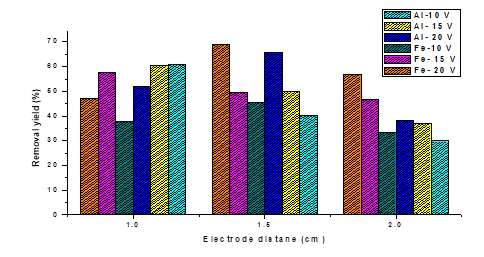I. INTRODUCTION
Drinking water treatment processes face great challenges in optimizing technologies to prevent the discharge of pollutants into the environment, which could cause problems in human health and environmental impact; this, due to continuous population growth, reduced availability of water sources, and climate changes, hydrology, and changes in water quality [1-2]. Emerging contaminants (EC), which comprise a wide range of chemicals, pharmaceuticals, personal care, surfactants, cosmetics, plasticizers, and industrial additives, have effect on health and the environment [3]. Surfactants are EC discharged into effluents from household cleaning, personal care products, and industries such as textiles, paints, polymers, pharmaceuticals, mining, oil recovery, pulp, and paper [4-5]. Among these, linear alkylbenzene sulfonate (LAS), is the most widespread anionic surfactant worldwide, widely used in detergents, personal care products, it can damage fish gills [6].
Due to their effects on aquatic biota, accumulation capacity and resistance to biodegradation, different technologies have been implemented in the removal of surfactants, such as bio-filtration, UV treatment, biological or oxidative treatments, and chlorination [7]. Therefore, the application of coagulation or electrocoagulation, are an alternative to the technologies conventionally employed in the removal of EPs [8]. Electrocoagulation is a technology that removes pollutants suspended, dissolved, or emulsified in water through electricity [1].
It has been used in the removal of sodium lauryl sulfate, achieving a 94.98 % of efficiency [8]; as well as in the removal of phosphates with iron and aluminum electrodes, finding that efficiency increases with voltage reaching 78 % removal [9], and in the dyes removal was achieved an efficiency of 97.57 % [10]. Electrocoagulation technology combines coagulation, flotation, and electrochemical processes in one cell; which contains electrodes, to generate coagulants and hydrogen gas bubbles to eliminate contaminants in wastewater [11]. The electrodes used are commonly made of aluminum (Al) or iron (Fe). When current is applied to the anodes, ions are released from the plate and dispersed in the bulk solution; these ions destabilize the particle suspension and form small flocs by decreasing the electrostatic repulsion between particles [12]. At the cathode, the water dissociates into OH- and H3O+ or H+, which produce hydrogen gas (bubbles) to lift the flocs to the top of the reactor, and then they are trapped in the foam [13]. Simultaneously, the cations (Al3+, Fe2+ or Fe3+) react with OH- ions to produce different species, such as Al(OH)2+, Al2(OH)2 4+, Al(OH)3, and charged cationic hydroxide complexes [11, 14-15].
In the water treatment by electrocoagulation, thus, the determination of the contaminant isotherm on the Aluminum electrode used is reported for Boron removal, considering that Faraday's Law corresponds to Langmuir's isotherm model [16]. Also, computer-aided modeling has been implemented using Response Surface Methodology (RSM) [17-18]. Thus, the objective of the present study was to design and evaluate the performance of a laboratory-scale electrocoagulation cell in greywater treatment, optimizing its operation by making changes in the electrode material, the distance between them, and the current supplied.
II. MATERIALS AND METHODS
In this investigation were used in the determination of the surfactant in water: Phenolphthalein as pH indicator, 1 N Sodium Hydroxide (NaOH) and 1 N and 6 N Sulphuric Acid (H2SO4) to adjust the pH of the solutions, Chloroform to preserve the samples, Methylene Blue, 30 % v Hydrogen Peroxide and Sodium Monobasic Phosphate monohydrate. All the reagents were of analytical grade. The remaining concentration in the solution was determined by spectrophotometry using a Shimadzu UV 1700.
A. Design of Experiments
The design of multilevel factorial experiments was implemented, considering as independent variables the distance between electrodes (1, 1.5 and 2 cm), the material of the sacrificial electrode (Aluminum or Iron) and the electrical voltage supplied to the system (10, 15, 20 V), and as a response variable the amount of surfactant removed (mg/L), for a total of 18 experiments. It was maintained constant the volume of solution (mL), pH, exposed area (cm2), current intensity (A), type of current (AC/DC), number of electrodes (10), electrode configuration (monopolar/bipolar), conductivity (µS/cm) and contact time (20 min).
B. Construction of the Electrocoagulation System
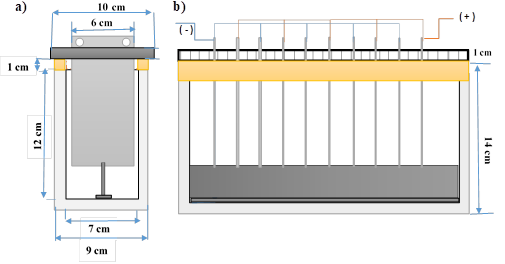
The electrocoagulation cell was built in 10 mm electrocoagulation glass with 26 cm x 7 cm base and 12 cm high; the foam area of the electrocoagulator was made by means of a PVC structure located on the upper edges of the walls except for the front wall of the cell.
C. Quantification of the Surfactant Solution
The determination of the surfactant concentration in the water was done following the S.M. 5540 C method, known as the Methylene Blue Active Substances (MBAS) method which allowed to know the concentration of LAS [19]. The concentration of LAS was determined by UV-VIS spectroscopy with a wavelength of 652 nm in a Biobase spectrophotometer model BK- UV1900.
D. Surfactant Removal by Electrocoagulation
Synthetic solutions were then prepared by adding 1.2, 0.6, and 0.3g of detergent per liter of solution, and the concentration was determined to be 203.45, 186.5, and 104.49mg/L, respectively. The value was taken from the solution with the closest value to the actual water (0.6 g of detergent/L). Table 1 shows the concentration values of the tests performed on synthetic greywater.
During the experiments carried out, we worked with a LAS solution at a concentration of 186 mg/L made from 2 L of water and 1.2 g of detergent. For each test, the electrocoagulation cell was filled with 1.8 L of the solution to be treated, and the electrodes were immersed, having a contact area of 48cm2 per electrode, giving a total of 480 cm2 of total area. The experimental tests were carried out following the experimental design; the operating voltage was set at 10 V. 1800 mL of gray water was placed in the electrocoagulation equipment for 20 minutes at pH 9.57, conductivity 815 µs, then the electrodes were removed and the solution was left to decant for one hour. To determine the concentration of LAS remaining in the solution after the process was completed, a 250 mL sample was taken from the water located in the center of the reactor in order not to remove or shake the sediments from the bottom. The above procedure was performed for the two electrodes evaluated (Al and Fe) by varying the electrical potential supplied to the system and the distance between electrodes. The percentage of surfactant removal was determined by Equation 1.
During the experiment, the initial and final pH, electrical conductivity, and temperature of the solution were measured. The evaluation of the effect of time on the removal efficiency was determined using the best configuration obtained within 60 minutes. Data (pH, conductivity, and temperature) were taken at 5, 10, 15, 20, 40, and 60 minutes.
III. RESULTS
This section presents the design and construction of the electrocoagulation cell, and the results of the LAS removal tests.
A. Design and Construction of the Electrocoagulation Cell
The reactor is firstly made up of the recipient, the electrical system, the electrodes, and the system for separating them. The length of the cell (CL) was calculated using the following equation 2 and 3 [4, 17].
Considering the number of electrodes (10), their thickness (0.1 cm), the largest distance between electrodes to be used (2 cm), and the distance between the first and last electrode on the front and back walls of the cell respectively (3.5 cm).
The width and length of the cell are 7 and 12 cm for a total capacity of 2184 cm3. The walls of the cell are made of glass with a thickness of 1 cm. A PVC structure was placed on the upper side and rear edges to act as a 1 cm high foam area. The dimensions are shown in Figure 1.
B. Effect of Electrode Material, the Distance Between Electrodes and Voltage
It was found that the highest removal obtained with the two metals is 65.55 % for aluminum and 69.11 % for iron, at the same evaluated conditions of voltage (20V) and electrode separation (1.5 cm). It has been reported that electrochemical reactors with aluminum sacrificial electrodes give better results in the removal of hardness, sulfates, phosphates, as well as wastewater and industrial water with turbidity and organic substances; while the iron was more efficient in the removal of inorganic substances, such as heavy metals, colored silica, fats and oils [20]. Another factor to take into account is the pH of the solution, which at the beginning is 9.57 on average and which increased when implementing electrocoagulation with iron electrodes, with 11.7 being the maximum value recorded, while with aluminum the pH does not vary significantly (Figure 2); this is a determining factor in electrocoagulation processes since it determines the electrochemical reactions in an aqueous medium [21].
The pH affects the solubility of the metal hydroxides and the predominant mechanism of the electrocoagulation process [22]. During electrocoagulation with iron electrodes can be generated various compounds depending on the conditions of the solution [23]. The distance between electrodes plays a fundamental role, since the amount of current is favored as the distance between electrodes decreases [24-25]; where the removal is higher with the shortest distance 1 cm, for voltage of 10 and 15 V for aluminum electrodes and 15 V for iron, for values of 20 V the best distance is 1.5 cm for both types of electrodes and the same for values of 10 V in iron electrodes.
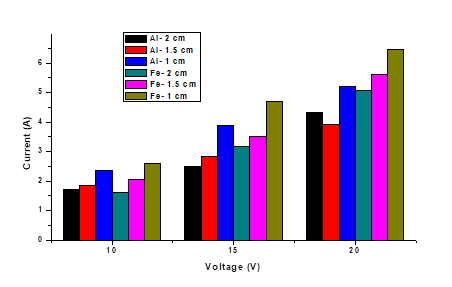
About the effect of voltage variation it can be said that, the higher the current, the greater the generation of metallic ions by the sacrificial electrode (anode), the production of hydrogen bubbles at the cathode increases as the current increases, which can help in the removal [26]. Expected experimental behavior is observed (Figure 3), where the highest removal is given with the highest voltage of 20 V and separation between electrodes of 1.5 cm, and the lowest removal values with voltages of 10 V and electrode separation 2 cm, for both types of the electrode (aluminum and iron). The highest percentage of removal was 65.55 % and 69.11 % for aluminum and iron respectively, the relationship between voltage and removal is directly proportional except when working with a 1 cm electrode separation where the highest removals were 60.99 % at 10 V for aluminum and 57.74 % at 15 V for iron.
C. Effect of Operating Time
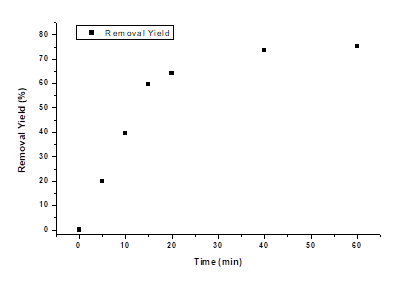
In Figure 4 illustrates the effect of time on LAS removal efficiency at the best configuration found (Al, 1.5 cm, and 20 V). The results coincide with previous studies, in which at times between 15 and 30 minutes, electrocoagulation reaches maximum removal capacity [20, 27]; however, in the present study, better removal efficiencies were obtained by increasing the operating time to 60 min.
The pH and conductivity increased, with values of 9.7 and 900 µs, due to the generation of aluminum hydroxide; after this, the conductivity began to drop to a value of 759 µs at 40 min and at 60 min it maintained that value because of the little removal that occurred at this interval. The current stabilized between minutes 10 and 20 with an average value of 3.73 A, then began to slowly decrease until it reached 3.2 A, due to the reduction in conductivity. The pH also fell to 9.21 at 15 minutes but increased to 9.47 at 20 minutes until it reached 9.99. This because the hydroxide carried away by the gases generated began to precipitate, increasing the pH. The notable reduction in the removal capacity of the electrocoagulator over time, was because the adsorption process involved in electrocoagulation depends on the concentration of the contaminant in the solution, so the efficiency decreases as the concentration does [28]. The final removal was 75.13 %, a significantly higher value than previous studies where removal of 35 % was obtained at pHs similar to those used but with a concentration of less than 100 mg/L [8].
IV. CONCLUSION
It was found that: (i) the increase in voltage affects directly proportional to the current and therefore to the removal; while the electrode separation has an inverse behavior. (ii) The best configuration was with 1.5 cm of electrode separation and a voltage of 20 V for the two types of aluminum and iron electrodes, with removal values of 65.55 % and 69.11 % respectively, in a working time of 20 min. (iii) The aluminum electrode showed better behavior than the iron electrode, with lower energy expenditure and without considerable effects on the pH of the solution. (iv) Increasing the time decreased the removal efficiency due to the lower availability of contaminant reduces the probability that this is trapped either in the form of a clot or being adsorbed by the clot, reaching removals of 73.67 % and 75.13 % in 40 and 60 min respectively.