Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista de Ciencias
Print version ISSN 0121-1935
rev. cienc. vol.19 no.2 Cali July/Dec. 2015
Comparison of DPPH Free Radical Scavenging, Ferric Reducing Antioxidant Power (FRAP), and Total Phenolic Content of Two Meriania Species (Melastomataceae)
Claudia Lorena Valverde Malaver
Departamento de Química, Universidad del Valle, Cali - Colombia
E-mail: claudia.valverde@correounivalle.edu.co
Ana Julia Colmenares Dulcey
Departamento de Química, Universidad del Valle, Cali - Colombia
E-mail: ana.colmenares@correounivalle.edu.co
José Hipólito Isaza Martínez
Departamento de Química, Universidad del Valle, Cali - Colombia
E-mail: jose.isaza@correounivalle.edu.co
Received: June 9, 2015
Accepted: November 5, 2015
Abstract
Searching for new antioxidants used in pharmaceuticals, cosmetics or agrochemicals have increased today. Many phenolic compounds have been reported as promising for this goal. Melastomataceae is rich in these compounds. Consequently, in this research the antioxidant power for Meriania nobilis and M. hernandoi ( Melastomataceae) was compared. Both species were extracted in acetone 70%. The latest was liquid-liquid partitioned in ethyl acetate (Mn or Mh 2.1), n-butanol (Mn or Mh 2.2) and aqueous fractions (Mn or Mh 2.3). Antioxidant activity was measured by DPPH free radical scavenging method (FRS50, mg.L-1), ferric reducing antioxidant power assay (FRAP, mg FeS04.7H20 (100 g)-1ES) and total phenolic content (TPC, mg AG g-1ES) by Folin-Ciocalteu. The present study revealed that Mh 2.1 and Mh 2.2 from M. hernandoi have a strong antioxidant power FRS50 = 5.71±0.085, FRAP = 53±1.5 and TPC = 224±7.1 and FRS50 = 4.22±0.001, FRAP = 65 mg±1.5, TPC = 240 ±8.6 respectively, as compared with positive control, quercetin DPPH FRS50 = 4.20±0.410, FRAP = 61±1.3. M. nobilis exhibited poor antioxidant power FRS50 = 286±1.5, FRAP = 2.01±0.079 and TPC = 26±1.5. This paper showed M. hernandoi as good source of antioxidant compounds with potential usage as cosmetic, pharmaceutical or agrochemical ingredients.
Keywords: Antioxidant power, DPPH free radical scavenging method (FRS), ferric reducing antioxidant power assay (FRAP), Estimation of total phenolic content (TPC), Meriania nobilis, Meriania hernandoi.
1. Introduction
Many studies have been published about phytochemicals extracted from plants with strong antioxidant activity. Compounds exhibiting this activity which are capable of neutralizing or eliminating free radicals and other reactive oxygen species (R0S) are generated in the body naturally (Tsao, Yang and Young 2003). An imbalance between oxidants and antioxidants in favor of oxidants develops the oxidative stress (Sies 1997). Oxidative stress is associated with an increased risk of some diseases such as diabetes, hypertension, obesity, dyslipidemia and inf ammation (Hopps, Noto, Caimi and Averna, 2010). Many phenolic compounds have been reported as promising secondary metabolites for this goal with similar activities to vitamins. The genera belonging to the family of Melastomataceae have been reported as plants rich in phenolic compounds such as favonoids (Mimura, Salatino and Salatino 2004, Rodrigues, Rinaldo, dos Santos and Villegas, 2007, Tarawneh, León, Ibrahim, Pettaway, McCurdy and Cutler, 2014), anthocyanins (Lowry, 1976), gallotannins, (Tan, Wong, Ling, Chuah and Kadir, 2012), hydrolyzable tannins, condensed tannins and ellagytanninos (Isaza 2007), having a range of biological activities: anti-infammatory (Murugan and Parimelazhagan, 2013), antimicrobial (Nono, Barboni, Teponno, Quassinti, Bramucci, Vitali, Petrelli, Lupidi and Tapondjou, 2014), antitumor, antibacterial (Yoshida, Amakura and Yoshimura 2010) and antioxidant (Gordon, Schadow, Quijano and Marx, 2011) activities.
Meriania is a neotropical genus, with more than 50 species, distributed in the Antilles, and from southern Mexico to southeast Brazil. In Colombia are known around 36 species, including Meriania nobilis and Meriania hernandoi, used mainly as ornamental. Their biological activities and phytochemicals have not been previously reported; therefore this research evaluated and compared antioxidant activity by DPPH free radical scavenging method (FRS), ferric reducing antioxidant power assay (FRAP) and correlation with total phenolic content (TPC) estimated by Folin-Ciocalteu method for both species, Meriania nobilis and Meriania hernandoi.
2. Materials and Methods
2.1 Plants and materials
Meriania nobilis Triana (Voucher CUVC-51735) was collected at Caldas, Antioquia, Colombia, on May 2012 and Meriania hernandoi L. Uribe (Voucher CUVC-62003) at "Reserva Civil El refugio", in Dagua, Valle del Cauca, Colombia, on November 2013. Both species, in fowering stage, were identifed in the herbarium "Luis Sigifredo Espinal Tascón" of the "Universidad del Valle".
2.2 Extraction
Dried and powdered leaves (500 g) of each species were successively extracted three times with hexane followed by acetone 70% in a Branson Scientifc ultrasonic bath at room temperature for 15 minutes. The solvent was evaporated at low pressure in an IKA RV10 Control V rotary evaporator at 40 oC; the latest was liquid-liquid partitioned in ethyl acetate (Mn or Mh 2.1), n-butanol (Mn or Mh 2.2) and aqueous fractions (Mn or Mh 2.3).
2.3 DPPH Radical - Scavenging Method
DPPH free radical scavenging activity was determined in triplicate by the method of Sdiri, et al. (Sdiri, Bermejo, Aleza, Navarro and Salvador 2012) with slight modifcations. Extracts (100 ¡L) at two-fold serial dilutions (0.5-512 mg.L-1) were mixed with 100 ¡L of a 132 mg.L-1 DPPH solution prepared in methanol. After 1 hour of reaction, the absorbance of the mixtures was read at 520 nm (Metertech, AccuReader M965+ microplate reader). Different concentrations (0.5-512 mg.L3) of vitamin C (ascorbic acid) and quercetin (Sigma-Aldrich) were used as positive controls and run in parallel. The results were expressed as a percentage of radical scavenging activity (%FRS) according to the equation:
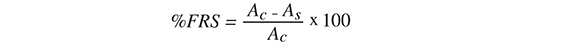
Where Ac is the absorbance of DPPH radicals without sample or positive control and As is the absorbance of DPPH radicals with sample or positive control. Effcient concentration of samples and positive controls that inhibits 50% of the DPPH radicals (FRS50) was calculated and expressed as mg. L-1.
2.4 Ferric Reducing Antioxidant Power Assay (FRAP)
Power reducing of Fe+3 to Fe+2 by an antioxidant was determined using the method reported by Benzie and Strain (Benzie and Strain 1996); this method was modifed for the 96 well microplate reader. Briefy, the FRAP reagent was prepared by mixing acetate buffer (300 mM, pH 3.6), 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) in 40 mM HCl, and 20 mM FeCl3 at 10:1:1 (v/v/v). All standards and samples were prepared at 1024 mg.L-1 in water or methanol. The 140 ¡L reagent, the 30 ¡L standard (FeS04.7H20) or sample solutions and 10 ¡L water were added to the well and conveniently mixed. The absorbance readings were taken at 600 nm immediately after and 60 min after using a Metertech, AccuReader M965+ microplate reader. A standard calibration curve of Iron (III) sulphate penta hydrate (FeS04.7H20) was plotted. Results were expressed as mg FeS04.7H20 (100 g)-1 dried extract.
2.5 Estimation of Total Phenolic Content (TPC)
Total phenolic content (TPC) were determined according to method of Sdiri, et al. (Sdiri, Bermejo, Aleza, Navarro and Salvador, 2012) with some modifcations. An appropriately diluted sample (100 ¡L) was mixed with 50 ¡L of 20% v/v Folin - Ciocalteu reagent (Sigma - Aldrich). Then, 50 ¡L of sodium carbonate solution (1,6% p/v) was added to the mixture. The mixture was incubated for 1 hour at 60 oC and then was allowed to stand at room temperature for 15 min. The absorbance was read at 700 nm. A standard calibration curve of gallic acid (1-32 mgL-1) was plotted. Results were expressed as gallic acid miligrams (mg AE) g-1 dried extract.
2.6 Statistical analysis
Results were given as the mean±SD for at least three replicates for each sample. Statistical analysis was performed using GraphPad Prism 5® (GraphPad software Inc., San Diego, California). FRS50 values were calculated using no nonlinear regression with one phase exponential decay calculation.
3. Results
The percentages of DPPH free radical scavenging (% FRS) for each extract from species M. nobilis and M. hernandoi were determined, as indicated in Figure 1; this fgure shows a free radical scavenging activity of 90% for M. hernandoi extracts.
Figure 1. Percentage of radical scavenging capacity for extracts of the species M. nobilis, M. hernandoi. Values are the average of three replicates
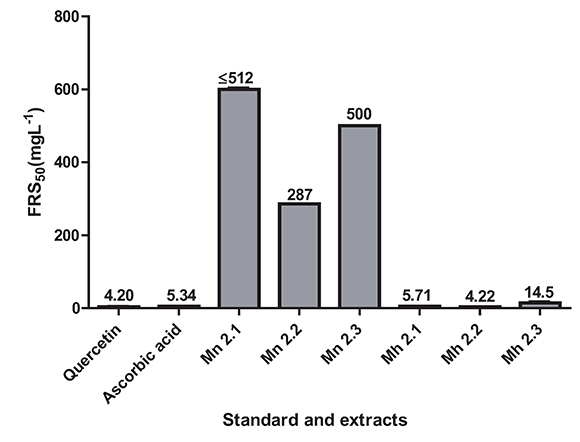
Also the concentrations of samples and positive controls that scavenge 50% of the DPPH free radicals (FRS50) were determined. The FRS50 values were ranging from the higher at 512 mg. L-1 (Mn 2.1) to the lowest at 4.22 mg. L-1 (Mh 2.2) as shown in the Figure 2. The extracts of M. nobilis showed a moderate radical scavenging activity, being Mn 2.2 the better with FRS50 equal to 287 ± 1.7 mg.L-1; while M. hernandoi showed strong radical scavenging in vitro in all extracts, Mh 2.1 (5.71 mg. L-1 ± 0.085), Mh 2.2 (4.22 mg. L-1 ± 0.001) and Mh 2.3 (14.5 mg. L-1 ± 0.119), similar to the values obtained for quercetin (4.20 mg. L-1 ± W0.410) and vitamine C (5.34 mg. L-1± 0.114) as the positive controls employed.
Figure 2. Activities radical scavenging for extracts of the species M. nobilis, M. hernandoi and positive controls. Values are the average of three replicates
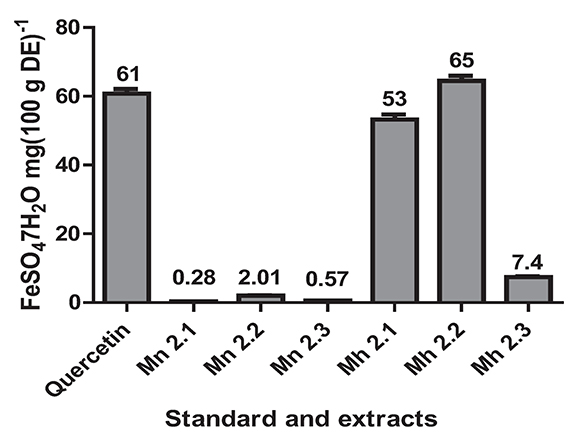
The antioxidant power was determined by the FRAP assay that measures the ability of the sample to reduce the Fe3+ in a solution of TPTZ to Fe2+. The values obtained for the samples ranged from 65 mg FeSO4.7H20 (100 g)-1ES to 0.28 mg FeSO4.7H20 (100 g)-1 ES (Figure 3). The M. hernandoi extracts that showed strong antioxidant power were Mh 2.1 (53 mg FeS04.7H20 (100 g)-1ES ±1.5) and Mh 2.2 (65 mg FeS04.7H20 (100 g)-1ES ±1.5). These values were very similar and even the Mh2.2 extract was greater than that for quercetin 61 mg FeSO4.7H20 (100 g)-1ES ±1.3. Very low values of antioxidant power were obtained for M. nobilis, such as Mn 2.1 (0.28 mg FeS04.7H20 (100 g)-1ES ± 0.009), Mn 2.2 (2.01 mg FeS04.7H20 (100 g)-1ES ± 0.079), and Mn 2.3 (0.57 mg FeS04.7H2O (100 g)-1ES ± 0.026).
Figure 3. The antioxidant power for extracts of the species M. nobilis, M. hernandoi and positive control. Values are the average of three replicates
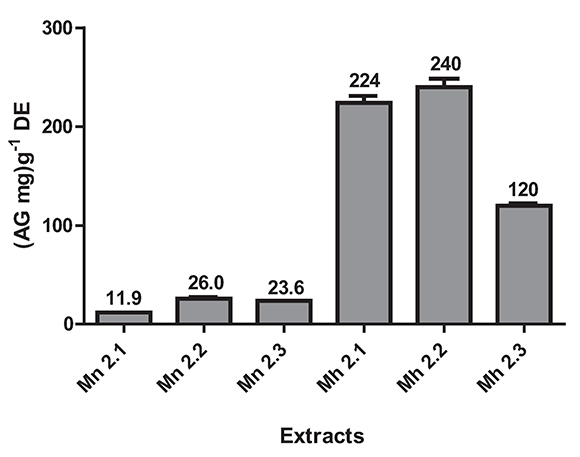
Total phenolic content estimation by Folin - Ciocalteu method was in the range from 11.9 to 240 (mg AG) g-1ES (Figure 4). The higher phenolic content was for M. hernandoi: Mh 2.1 (224 (mg AG) g-1ES ± 7.1), Mh 2.2 (240 (mg AG) g-1ES ± 8.6), and Mh 2.3 (120 (mg AG) g-1ES ± 2.5).
Figure 4. Estimation of total phenolic content by Folin-Ciocalteu method of the species M. nobilis and M. hernandoi. Values are the average of four replicates
4. Discussion
The results obtained for % FRS showed that M. Hernandoi extracts possess a striking antiradical activity of 90%, while for the M. nobilis extracts the percentages were obtained from 10 to 60%. These results are also supported by the great correlations between DPPH FRS50 with FRAP (R2 = 0.9974), DPPH FRS50 with TPC (R2 = 0.901), and TPC with FRAP (R2 = 0.9950) as shown in the Figure 5. FRAP values obtained for the two species confrmed the values found in FRS50 assay, showing to M. hernandoi extracts, specially Mh 2.1 and Mh 2.2 as good source of antioxidant compounds from natural origin with potential usage as cosmetic, pharmaceutical or agrochemical ingredients.
Figure 5. Correlations between the methods used to determine antioxidant activity. FRS50= DPPH free radical scavenging 50 , Effcient cfncentratifn that inhibits 50% ff the DPPH radicals FRAP= Ferric Reducing Antioxidant Power, TPC= Total Phenolic Content
Therefore, extracts exhibiting strong radical scavenging capacity (FRS) and a high antioxidant power (FRAP) also have higher levels of total phenolic content (TPC), suggesting phenolic compounds as potential responsible for this activity. The values of FRS50 and FRAP for the two species behaved similarly. This was checked by determining the correlation coeffcient for the two methods, which was: R2 = 0.9974; (Y= (655-0.52) e0.423X -0.52; 95% confdence intervals; 3 degrees of freedom), indicating a statistically signifcant correlation. Also the correlation coeffcients were determined for the values obtained FRS50 and TPC and FRAP and TPC. The correlation coeffcients obtained were: R2 = 0.9010; (Y= (919-2.65) e0.034X -2.65); R2 = 0.9950; (Y= (8.26-2.36) e0.423X -2.36); 95% confdence intervals; 3 degrees of freedom; respectively (Figure 5). A statistically signifcant correlation for the methods is observed.
Many in vitro methods are used to determine the antioxidant activity, but they do not correlate well with each other (Tsao, Yang and Young, 2003); Although in this research methods with different mechanisms were used, they correlate really well, indicating that the main compounds responsible of both antioxidant activities, DPPH free radical scavenging and ferric reducing antioxidant power of M. nobilis and M. hernandoi, are phenolics.
This paper showed the species M. hernandoi as good source in antioxidant compounds from of natural origin with potential usage as cosmetic, pharmaceutical or agrochemical ingredients, due to activity found by the methods in vitro free radical DPPH scavenging, ferric reducing power antioxidant assay (FRAP) and total phenolic content. The extracts of M. hernandoi are promising for future studies to isolate and elucidate the metabolites that exhibit this activity. The specie Meriania nobilis showed a poor antioxidant activity, which agree to in vitro methods employed. The in vitro method employed correlated well which allowed determining the antioxidant power of the two species studied.
Acknowledgements
Authors are grateful to COLCIENCIAS, Grant CT-410-2011, and to Universidad del Valle Grant CI 7848 and professor Carlos Calderon, of the "Reserva Civil El Refugio" by providing plant material.
References
Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Analytical Biochemistry, 239, 70-76. [ Links ]
Gordon, A., Schadow, B., Quijano, C. E., & Marx, F. (2011). Chemical characterization and antioxidant capacity of berries from Clidemia rubra (Aubl.) Mart. (Melastomataceae). Food Research International, 44, 2120-2127. [ Links ]
Hopps, E., Noto, D., Caimi, G., & Averna, M. R. (2010). A novel component of the metabolic syndrome: The oxidative stress. Nutrition, Metabolism and Cardiovascular Diseases, 20, 72-77. [ Links ]
Isaza, J. H., Veloza, L. A., Ramírez, L. S., y Guevara, C. A. (2007). Estimación espectrofotométrica de taninos hidrolizables y condensados en plantas melastomatáceas. Scientia Et Technica, 13, 5. [ Links ]
Lowry, J. B. (1976). Anthocyanins of the Melastomataceae, Myrtaceae and some allied families. Phytochemistry, 15, 513-516. [ Links ]
Mimura, M. R. M., Salatino, A., & Salatino, M. L. F. (2004). Distribution of favonoids and the taxonomy of Huberia (Melastomataceae). Biochemical Systematics and Ecology, 32, 27-34. [ Links ]
Murugan, R., & Parimelazhagan, T. (2013). Study of anti-nociceptive, antiinfammatory properties and phytochemical profles of 0sbeckia parvifolia Arn. (Melastomataceae). Industrial Crops and Products, 51, 360-369. [ Links ]
Nono, R. N., Barboni, L., Teponno, R. B., Quassinti, L., Bramucci, M., Vitali, L. A. et al. (2014). Antimicrobial, antioxidant, anti-infammatory activities and phytoconstituents of extracts from the roots of Dissotis thollonii Cogn. (Melastomataceae). South African Journal of Botany, 93, 19-26. [ Links ]
Rodrigues, J., Rinaldo, D., dos Santos, L. C., y Vilegas, W. (2007). An unusual linked favonoid from Miconia cabucu (Melastomataceae). Phytochemistry, 68, 1781-1784. [ Links ]
Sdiri, S., Bermejo, A., Aleza, P., Navarro, P., & Salvador, A. (2012). Phenolic composition, organic acids, sugars, vitamin C and antioxidant activity in the juice of two new triploid late-season mandarins. Food Research International, 49, 462-468. [ Links ]
Sies, H. (1997). 0xidative stress: oxidants and antioxidants. Experimental Physiology, 82, 291-295. [ Links ]
Tan, H. P., Wong, D. Z. H., Ling, S. K., Chuah, C. H., & Kadir, H. A. (2012). Neuroprotective activity of galloylated cyanogenic glucosides and hydrolysable tannins isolated from leaves of Phyllagathis rotundifolia. Fitoterapia, 83, 223-229. [ Links ]
Tarawneh, A. H., León, F., Ibrahim, M. A., Pettaway, S., McCurdy, C. R., & Cutler, S. J. (2014). Flavanones from Miconia prasina. Phytochemistry Letters, 7, 130-132. [ Links ]
Tsao, R., Yang, R., & Young, J. C. (2003). Antioxidant Isofavones in 0sage 0range, Maclura pomifera (Raf.) Schneid. Journal of Agricultural and Food Chemistry, 51, 6445-6451. [ Links ]
Yoshida, T., Amakura, Y., & Yoshimura, M. (2010). Structural features and biological properties of ellagitannins in some plant families of the order myrtales. International Journal of Molecular Sciences, 11, 79-106. [ Links ]

Revista de Ciencias por Universidad del Valle se encuentra bajo una licencia Creative Commons Reconocimiento 4.0.