1 Introduction
Hydrazones have become in an important type of compounds for supramolecular chemistry due to the facile synthesis, ability to coordinate metal ions and the ability of interact with UV light to undergoing configurational changes 1-8. Special attention has been paid to double hydrazones containing the framework pyridine-pyrimidine-pyridine since these compounds are able of double coordinate metal ions in a terpyridine-like manner. 5,6 Taking in account such characteristic, it has been prepared a great variety of complexes, among them grid-type complexes have attracted great interest not only for their remarkable architecture but also for its potential applications in molecular electronics, spintronics and molecular magnetics. 1-16
In contrast of the aforementioned properties, electrochemical studies of hydrazone based metallogrids or linear complexes of double hydrazones are scared. 6-9 Therefore, herein we present the synthesis of the 2-phenylpyridimine-4,6-yl- bis-(hydrazine-2-yl-1-ilydine- bis- (metaniylinde)dypynacolic acid, a bis-(hydrazone) able of forming metallogrids and linear complexes with Zn(II), Cd(II) and Pb(II). Cyclic voltammetry and squared wave voltammetry were used to study the redox properties of the complexes. We also examined the temperature dependence of the redox potentials.
2 Experimental Section
All starting reagents were acquired from Sigma-Aldrich and were used without additional purification. The FT-IR, NMR (mono and bi-dimensional), UV-vis, and fluorescence spectra were taken in Shimadzu FTIR-8400 spectrophotometer, NMR 400 MHz Bruker Ultra Shield, and Pharma Spec Shimadzu UV-Vis UV-1700 spectrophotometer, respectively. The mass spectra were obtained on a Hewlett Packard HP Engine-5989 spectrometer (equipped with a direct inlet probe) operating at 70 eV. The elemental analyses were obtained using a Thermo-Finnigan Flash EA1112 CHN (Elemental Microanalysis Ltd, Devon, UK) elemental analyzer.
2-formylpynacolic acid
To a 50 mL two-neck round bottom was added 6-methylpyridin- 2-carboxilic (100 mg, 0.73 mmol) and selenium dioxide (161 mg, 1.46 mmol) followed by 25 mL of Dioxane:Water (4:1). The resulting mixture was heated to reflux for 4 h. The reaction was allowed to cool down and the solid formed was filtered and washed with chloroform (3x2 mL). The filtrates were combined and the solvent removed under reduced pressure. Column chromatography using silica gel and CHCl3: MeOH ([95:5]) afforded the desire product 5 (57 mg, 54%). FT-IR (KBr) υ (cm-1): 3100 (=C-H), 2910 (=C-H), 1740 (C=O), 1700 (C=O), 1580 and 1438 (C=N and C=C). 1H NMR (400 MHz, CDCl ) δ ppm: 10.18 (s, 1 H, CHO), 8.51 (d, J = 4.78 Hz, 1 H, H-5), 8.27 (d, J = 4.78 Hz, 1 H, H-5), 8.20 (m, 1 H, H-4). 13C NMR (100 MHz, CDCl3) δ ppm: 191.5, 163.1, 151.28, 146.8, 139.83, 128.07, 125.45. Elemental analysis calculated (%) for C8H12N2O: C 61.75, H 5.92, N 20.58; found: C 61.62, H 5.84, N 20.13.
4,6-dihydrazino-2-phenylpyrimidine
To a 50 mL two-neck round bottom was added 4,6-dichloro-2-phenylpyrimidine (100 mg, 0.44 mmol) and ethanol 25 mL followed by hydrazine monohydrate (0.043 mL, 0.89 mmol). The resulting mixture was heated to reflux for 3 days. The reaction was allowed to cool down and concentrated under reduced pressure. The hydrazine excess was removed by decantation obtaining a solid. Recrystallization from methanol afforded the desire product 4 (62 mg, 64%) as a white solid. m.p.: 142 ºC. 1H NMR (400 MHz, CDCl ) δ ppm: 8.26 (m, 2 H, NH), 7.41 (m, 3 H, H-5), 6.74 (s, 2H, H-X), 5.81 (s, 1 H, H-pyr), 3.7 (brs, 4H, NH ).13C NMR (100 MHz, CDCl3) δ ppm: 166.6, 163.3, 138.3, 130.0, 128.2, 127.9, 78.0. MS (70 eV) m/z (%) 216 (16), 164 (23), 103 (100), 46 (58). Elemental analysis calcd (%) for C10H12N6: C 55.54, H 5.59, N 38.86; found: C 56.10, H 5.50, N 39.09.
2-phenylpyridimine-4,6-yl-bis-(hydrazine-2-yl-1-ilydine-bis-(metaniylinde) dypynacolic acid
To a 50 mL neck round bottom was added 2-formylpynacolic acid (161 mg, 1.46 mmol) and 4,6-dihydrzin-2-phenylpyrimidine (72 mg, 0.33 mmol) and followed by 25 mL of methanol. The resulting mixture was heated to reflux for 3 h. The reaction was allowed to cool down and the solid formed was filtered and washed with ethanol (3x2 mL). Recrystallization from methanol afforded the desire product 1 (131 mg, 82%) as a white solid. m.p. 242º C.1H NMR (400 MHz, CDCl ) δ ppm: 11.68 (s, 2 H, N-H), 8.34 (t, 2 H, H-3), 8.27 (s, 2 H, H imino), 8.24 (dd, 2 H, H-10), 8.09 (t, 2 H, H-11), 8.00 (dd, 2 H, H-12), 7.51 (m, 3 H, H-1, H-2), 6.99 (s, 1 H, H-7). 13C NMR (100 MHz, CDCl3) δ ppm: 163.4, 162.7, 154.3, 148.8, 141.9, 18.6, 138.2, 10.8, 128.7, 128.0, 124.8, 123.0, 81.3. Elemental analysis calculated (%) for C24H18N8N4: C 59.75, H 3.75, N 23.23; found: C 59.10, H 3.50, N 22.89.
Zn Complex [Zn(C 24 H 18 N 8 N 4 )( CF 3 SO 3 ) 4
To a 50 mL neck round bottom was added 1 (100 mg, 0.21 mmol) and 40 mL of acetonitrile followed by 2 mL methanolic solution of Zn(OTf)2 (158 mg, 0.44 mmol). The resulting mixture was heated to reflux for 4 h. The reaction was allowed to cool down and concentrated under reduced pressure. The solid formed was filtered and washed with ether (3x2 mL) afforded the desire product 6a (205 mg, 82%) as a white solid. m.p.: 242 ºC. 1H NMR (400 MHz, CDCl ) δ ppm: 11.69 (s 2H, N-H), 8.36 (m 2H, H-3), 8.29 (s 2H, Himidine), 8.27 (m 2H, H-10), 8.11 (t 2H, H-11), 8.03 (d 2H, H-12), 7.54 (t 3H H-1 and H-2),7.02 (s 1 H, H-7). Elemental analysis calcd (%) for C28H18F12N8O16S4Zn2: C 27.81, H 1.50, N 9.26; found: C 27.4, H 1.64, N 9.13.
Cd Complex [Zn(C 24 H 18 N 8 N 4 )( CF 3 SO 3 ) 4
To a 50 mL neck round bottom was added 1 (100 mg, 0.21 mmol) and 40 mL of acetonitrile followed by 2 mL methanolic solution of Cd(OTf)2 (174 mg, 0.42 mmol).. The resulting mixture was heated to reflux for 3 h in atmosphere inert. The reaction was allowed to cool down and concentrated under reduced pressure. The solid formed was filtered and washed with ether (3x2 mL) afforded the desire product 6b (183 mg, 68%) as a brown solid. m.p.: 242º C. 1H NMR (400 MHz, CDCl ) δ ppm: 11.69 (s 2H, N-H), 8.36 (m 2H, H-3), 8.29 (s 2H, Himino), 8.27 (m 2H, H-10), 8.11 (t 2H, H-11), 8.03 (d 2H, H-12), 7.54 (t 3H H-1 and H-2),7.02 (s 1 H, H-7). Elemental analysis calculated (%) for C28H18F12N8O16S4Cd: C 25.80, H 1.39, N 8.60; found: C 25.11, H 1.66, N 8.37.
Pb Complex [Pb(C 24 H 18 N 8 N 4 )( CF 3 SO 3 ) 4
To a 50 mL neck round bottom was added 1 (100 mg, 0.21 mmol) and 40 mL of acetonitrile followed by 2 mL methanolic solution of Zn(OTf)2 (262 mg, 0.52 mmol). The resulting mixture was heated to reflux for 3 h in atmosphere inert. The reaction was allowed to cool down and concentrated under reduced pressure. The solid formed was filtered and washed with ether (3x2 mL) afforded the desire product 6c (241 mg, 78%) as a brown solid. m.p.: 242º C. 1H NMR (400 MHz, CDCl3) δ ppm: 11.69 (s 2H, N-H), 8.36 (m 2H, H-3), 8.29 (s 2H, Himino), 8.27 (m 2H, H-10), 8.11 (t 2H, H-11), 8.03 (d 2H, H-12), 7.54 (t 3H H-1 and H-2),7.02 (s 1 H, H-7).
Elemental analysis calculated (%) for C28H18F12N8O16S4Pb2: C 25.80, H 1.39, N 8.60; found: C 25.11, H 1.66, N 8.37.
Pb Complex 7c, [Pb 4 (14) 4 ](CF 3 SO 3 ) 8
to a solution of 1 (100 mg, 0.21 mmol) in ethanol (40mL) was added 1 mL solution 1 M of Pb(OTf)2 in methanol. The reaction mixture was heated for 4 h in Ar atmosphere. The solvent was removed by pressure reduced resulting a brown solid, which was washed with ether (3x2 mL) afforded the desire product 7c (1.5 g, 72%). 1H NMR (400 MHz, CDCl ) δ ppm: 11.70 (s 2H, N-H), 8.36 (m 2H, H-3), 8.30 (s 2H, Himine; m 2H, H-10), 8.10 (t 2H, H-11), 8.05 (d 2H, H-12), 7.53 (t 3H H-1 and H-2),7.03 (s 1 H, H-7). Elemental analysis calculated (%) for C128H144F24N32O40Pb4S8: C, 35.65; H, 3.37; N, 10.39; found: C, 34.50; H, 3.55; N, 10.18.
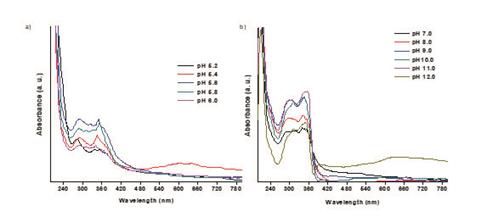
pH-Dependence of hydrazone derivative 1
To an aliquot of the solution of 1 was diluted in solutions of known pH and the UV-vis spectra were recorded. The buffer solutions at pH between 2 and 12 were prepared according to the literature 3 (Table 1).
Table 1 Buffer systems used for obtaining the required pH.
| Buffer pH | System | Obtained pH |
|---|---|---|
| 2 | KCl + HCl | 2.07 |
| 3 | KC8H5O4 + HCl | 3.10 |
| 4 | KC8H5O4 | 4.05 |
| 5 | KC8H5O4 + NaOH | 5.03 |
| 6 | KH2PO4 + NaOH | 6.15 |
| 7 | KH2PO4 + NaOH | 7.08 |
| 8 | KH2PO4 + NaOH | 8.08 |
| 9 | H3BO3 + KCl + NaOH | 9.01 |
| 10 | H3BO3 + KCl + NaOH | 10.12 |
| 11 | Na2HPO4 + KCl + NaOH | 11.03 |
| 12 | KCl + NaOH | 12.05 |
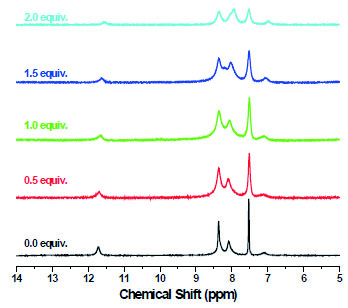
Deprotonation Study of 7c
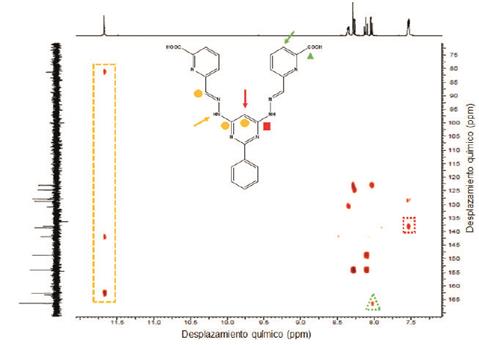
To a solution of 7c in DMSO-d6 was added 0.5 equivalents of a solution 4% of NaOD in deuterated water and NMR spectra were immediately recorded. The procedure was repeated until add 2.0 equivalents of NaOD.
Electrochemical study of metal complexes
The measurements were performed using a 0.1 M solution of tetrabutylammonium hexafluorophosphate in acetonitrile. It was employee a glassy carbon electrode, a platinum wire and a silver wire as working; counter electrode and pseudo-reference electrode respectively. The solutions were degassed before each measurement and added at the end of ferrocene as an internal standard measures. The scan rate was 100 mV/s. The electrochemical properties were measured at 25° C and -10° C.
3 Results and discussion
Synthesis and purification of bis-(hydrazone) 1
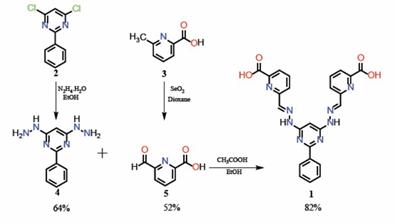
Hydrazone derivative 1 was prepared in a multistep synthetic procedure. The first step required the preparation of hydrazine and aldehyde derivatives 4 and 5 as shown in Scheme 1. Compound 2 was obtained as reported by Lehn´s group 10-16 and immediately used to form the hydrazine 4 in a 64 % yield. On the other hand, the partial oxidation of the methyl group of 6-methyl-2-pyridinecarboxylic acid (3) with SeO2 led to the formation of the aldehyde derivative 5 in a 52 % yield. The corresponding bis-(hydrazone) derivative 1 was synthesized by condensation of 4 with 5 (2 equivalents) in reflux of ethanol with the addition of catalytic amounts of glacial acetic acid, obtaining exclusively the E-isomer.
Scheme 1. Synthesis of bis-(hydrazone) 1.
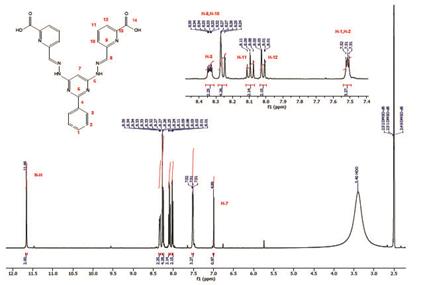
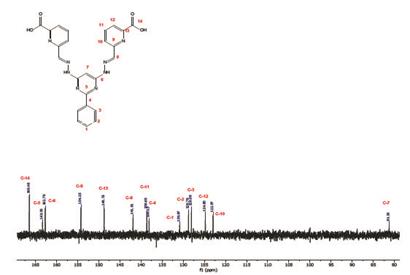
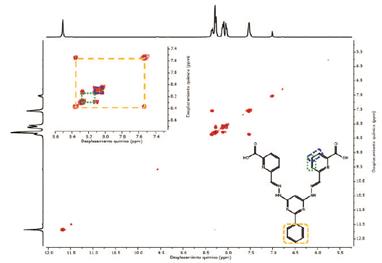
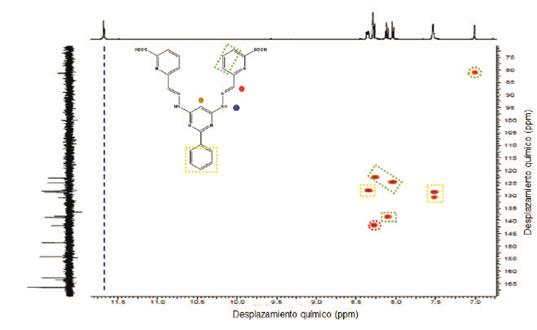
Compounds 4, 5 and 1 were characterized by 1H, 13C and COSY NMR, FT-IR, and elemental analysis (see experimental section and supporting information). 1H-NMR of compound 1 exhibits 8 of the 9 expected signals, the carboxylic proton is not observed due to fast proton exchange with the solvent. The -N-H protons appears at 11.66 ppm as other protons of E configuration found in the literature 3-16. The imine proton is observed at 8.27 ppm and the pyrimidine (C7) proton is found at 6.99 ppm. 13C-NMR shows 14 signals corresponding to the equivalent carbons of the framework (see supporting information). Assignment of the signals was based on COSY and by comparison with other bis- (hydrazone) analogues found in the literature.
pH-dependence of bis-(hydrazone) 1
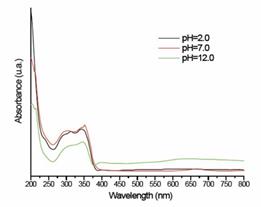
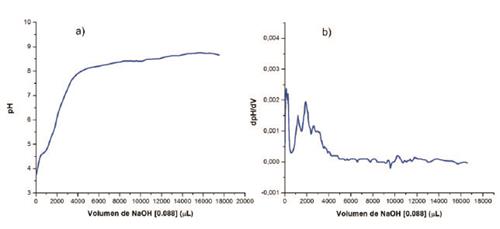
Compound 1 contains two different acidic protons (carboxylic and amine groups), thereby the study of the behavior at different pH´s represent an effective tool in order to determine the influence of the acidic protons on the electronic properties of the hydrazone framework 1,8. Titration with a NaOH solution allowed us to find two equivalence points at pH 3.82 and 5.86, which correspond to the carboxylic and the N-H protons, respectively (see supporting information). Additionally, UV-Vis spectra were recorded at different pH´s ranging from 2.0 to 12.0. For simplicity only UV-vis spectra of 1 at pH 2.0, 7.0 and 12.0 is shown in Figure 1. Increasing the pH above 6.0 causes a bathochromic shift on the π-π* transition bands, such transitions are associated to the imine bond with wavelengths between of 260-370 nm. 5 This shift was also observed when the solvent was replaced by methanol (see Supporting information). Noteworthy, the appearance of bands in the visible region when the pH is increased; this is related to deprotonation of the acid protons which causes an increase of electron delocalization and therefore a decrease in the energy of the π* molecular orbital.
Synthesis and characterization of metal complexes
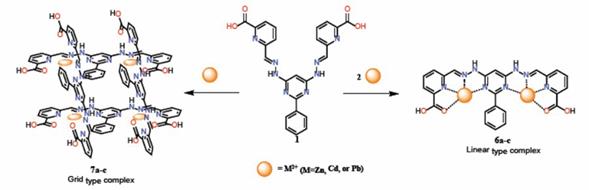
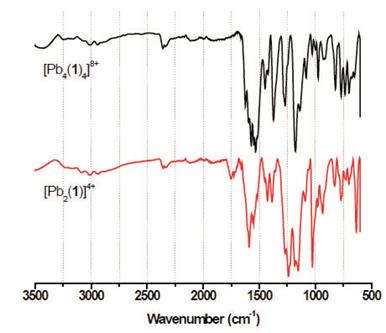
Metal complexes were obtained through the reaction of 1 with transition divalent metal salts (ZnII, CdII, and PbII) in methanol in a stoichiometric ratio 1:2 (Scheme 2). It has been known that bis-(hydrazones) show a transoid conformation, 6-16 however, formation of metal complexes requires a conformational change in the molecule from transoid to cisoid6,7. After precipitation, the complexes were washed with ethyl ether and cold ethanol with yields between 68-78 %. These compounds were characterized by FT-IR and NMR spectroscopy and elemental analysis (see experimental section).
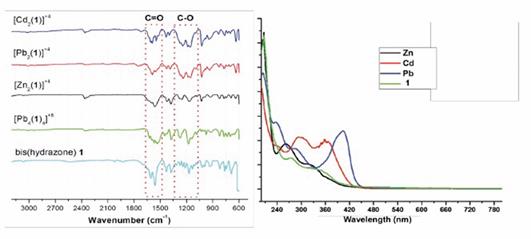
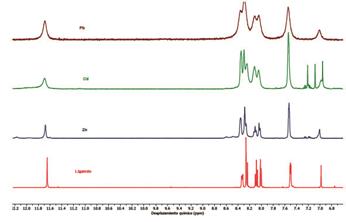
As observed in the FT-IR spectra shown in Figure 2, complexation of 1 with metal ions causes a shift in the N-H and double bonds (C=C and C=N) bands towards more and less energetic regions, respectively, which confirms the formation of the complexes 6a-c. Such shift is due to the increase of the ionic radius of the metal which provokes a shift in these frequencies.17 It can be also observed shift and widening in some signals depending on the nature of the metal ion due to the formation of the metal complex (see supporting information).
On the other hand, the UV-vis spectra shows an increase in the maximum absorption band which is accompanied by the appearance of new bands in the visible region corresponding to the metal d-d absorptions. Additionally, the increase of the ionic radius results in a decrease in the spectral onset (see supporting information). 3-5
Alternatively, the 1H-NMR spectra shows a marked dependence of the chemical shifts on the nature of the metal ions present in the complex. Widening of some signals is due to the unpaired electrons in the metal ions, which causes significantly changes on the local magnetic field (Figure 3). Noteworthy, NMR is not conclusive about the nature of the complexes and therefore it is not possible to determine a possible structure. However, the molecular structure of the complexes was corroborated by elemental analysis in all cases.
We planned to synthesize metal-grids [2x2] changing the molar ratio of 1 and metal ions (1:1) as depicted in Scheme 2. Nevertheless, when the reaction was carried out with Zn2+ and Cd2+ only the linear complexes were obtained with. The resistant to yield grid- like complexes is explained by the small ionic radii of these metals (88 and 109 pm, for Zn2+ and Cd2+, respectively) 18 and the also preference to coordinate in a tetrahedral manner. It is important to remember that compound 1 is able to coordinate in both arms through four atoms, three nitrogen atoms of the pyridine-imine and pyrimidine framework and a fourth one from the carboxylic group, thus the four sites of coordination prefer to generate a more stable tetrahedral complex around the ion (Zn2+ or Cd2+) than a complex of coordination 8 that would have in a grid-type complex. On the other hand, Pb2+ has an ionic radius of 133 pm and prefers the octahedral coordination, which facilitates to obtain metalo-grids 11,19.
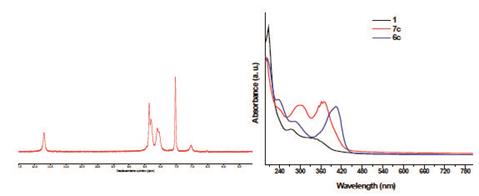
As shown in the Figure 4a, the 1H-NMR spectrum showed a widening of the signals caused by the diamagnetic effect provoked by the lead atom on the external magnetic field of NMR. Besides, the UV-vis spectrum also showed a bathochromic shift in the main absorption peak respect to 1 due to the charge transference between the metal center and ligand (Figure 4b). While, 7c exhibits an increasing on the absorption peak when compared with 6c.
Electrochemical studies of metal complexes
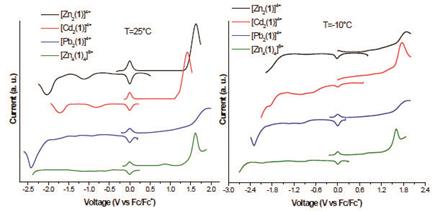
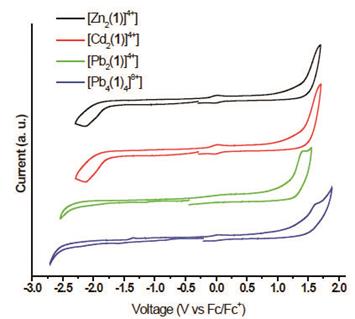
The electrochemical properties of compounds 6a-c and 7c were studied by cyclic voltammetry (CV) and Osteryoung Square Wave Voltammetry (OSWV). The complexes exhibit irreversible oxidation peaks corresponding to hydrazone group. The complexes also presented two irreversible reduction peaks which correspond to the reduction of the imine group and to the M2+/M+ events. If we compare the Pb2+ complexes (linear vs grid) an anodic shift of 260 mV is observed, being easier to oxidize the grid. While the reductions showed a cathodic shift in the first reduction potential and an anodic shift in the second reduction.
On the other hand, the Cd2+ and Zn2+ complexes showed a similar behavior, where the Cd complex is oxidized and reduce easier than its counterparts (Table 2). We also investigated the temperature effect on electrochemical properties of these compounds. Thus, we carried out the electrochemical studies at -10° C. However, we found that the decrease of temperature has not a significant effect on the number of peak potentials of oxidation-reduction. In general, we can say that a lowering of the temperature causes a small anodic shift on both oxidation ad reduction potentials.
Table 2 Redox Potentials of compounds 6a-c and 7c vs Ferrocene in acetonitrile (V)a
| Compound | E p ,red 1 | E p ,red 2 | E p ,ox 1 |
|---|---|---|---|
| 6a → [Zn2(1)]4+ | -1.12 (-0.86) | -2.04 (-1.93) | 1.62 (1.88) |
| 6b → [Cd2 (1)]4+ | -0.83 (-0.75) | -1.71(-1.86) | 1.40 (1.76) |
| 6c → [Pb2 (1)]4+ | -0.93(-1.11) | -2.42(-2.29) | 1.87 (1.87) |
| 7c → [Pb4 (1) 4]8+ | -1.03(-0.99) | -2.01(-2.10) | 1.61 (1.60) |
aWorking electrode: glassy carbon;
counter electrode: Pt wire;
Pseudoreference electrode: Ag wire.
Supporting electrolyte: TBAPF6
Scan rate: 0.1 V s-1.
3. Conclusions
We have synthesized a bis-(hydrazone) derivative bearing two carboxylic groups. This compound contains two type of acidic protons whose equivalent points were found at pH 3.82 y 5.86. The bis-(hydrazone) is able to change its conformation from transoid to cisoid upon addition of metal ions yielding different complexes. Electronic and electrochemical properties were studied thought UV-Vis spectroscopy and cyclic and squared wave voltammetry.