1 Introduction
Man has always been in a constant interaction with other organisms, mainly with domestic animals like dogs, cats, rodents, cattle, some birds, and others. However, despite the benefits of having a group of animals like these, there are certain risks usually related to infectious agents or pathogens (such as bacteria, fungi and parasites) that can be found in animals mentioned before 1. Bacteria and fungi that are potentially pathogenic to humans are just a small portion of the total of bacteria and fungi known, which can cause various diseases with specific or varied symptoms 2,3, domestic animals can be reservoirs for these microorganisms through environmental exposition, commensal opportunistic bacteria and fungi or a foreign agent 2. Bacteria can be transmitted mainly through direct contact with animal feces or some type of fluid 1 . In the case of fungi transmission, it occurs mostly through the contact with animal’s skin or hair 3. Also green areas or parks, where people attend with their pets (usually dogs) are considered an important part in the dynamics of the city population, as they are constantly visited areas 4. These zones are mainly made up of areas with vegetation and recreative elements which act as fomites and implies direct or indirect interaction with, for example, pets and possibly their depositions, which in many cases are not handled in a proper way, being left in the area where people and other dogs are exposed to them.
Some bacteria and fungi related to pets or domestic animals that are capable of being transmitted from these and cause human diseases are Escherichia coli, Salmonella spp, Shigella spp, Yersinia spp, Microsporum spp, among others 5 - 7, which are often associated with symptoms like fever, chills, muscle pain, diarrhea, vomiting or nausea, tinea (mainly capitis), among others6 , 7. Treatment of diseases caused by bacterial infections usually required antibiotics, which have different mechanisms of action, considering that each type of antibiotic affects a certain group of bacteria as Gram-positive bacteria or Gram- negative bacteria, or even both 2 , 8 , 9.
Some groups are more vulnerable than others such as children, elderly people and immune compromised individuals 10,11. People usually don’t know that they can be exposed to a big number of microorganisms in a park or green areas where dogs attend and recreational elements and plant material are in constant contact with lots of people and other animals, which can lead to a public health problem where a focus of infection could be generated, unknowing what produce it and how can it can be avoided 5 , 7. In Colombia there are few publications on this subject, most of them are mainly focused on zoonosis related with protozoa, helminths, nematodes and other parasites in dogs and cats 12-14. That’s why it is planned to contribute to the knowledge with the identification of bacteria and fungi found in feces, fur of dogs, recreational elements and vegetation in some public parks in the city of Bogotá D.C, Colombia.
2 Materials and methods
2.1 Parks selection
We selected at northern El Virrey Park, at southern Timiza Park, at eastern Nacional Park and at western Simon Bolívar Park. In each one, four items were sampled: fecal samples, canine fur, environment samples that were near populated zones and elements in recreational areas. A total of 50 samples were taken distributed in ten samples for each park except on Simon Bolivar Park where twenty samples were collected because its bigger extension.
2.2 Cultures procedure and biochemical testing
2.2.1 Fecal samples
were cultivated on McConkey or Eosine Methylene Blue agar during 24 hours at 37° C then the macroscopic description was done followed by the culture of the following biochemical test: for Gram-negative bacteria were used Triple Sugar Iron agar, Citrate, Indole (SIM Medium), Urease , Voges-Proskauer and Methyl Red tests. After the identification of bacteria, we performed the Kirby-Bauer test, ampicillin, ceftriaxone, ciprofloxacin trimethoprim, gentamicin for Gram-negatives and oxacillin, clindamycin, ciprofloxacin, trimethoprim and gentamicin for Gram-positives cocci. This procedure was applied to bacteria isolated from other types of samples.
2.2.2 Recreational areas samples
Were taken to Blood Agar and were incubated for 48 hours at 37°C, followed by macroscopic description and Gram stain. For Gram-positives cocci were performed catalase test and for Gram-positive rods were no further tests performed. Catalase positive bacteria were cultured in Mannitol Salt Agar and coagulase test; in some cases where test were undetermined we performed agglutination test for Staphylococcus aureus.
2.3 Identification
For Gram-negative bacteria, identification was done using macroscopic description and biochemical test, Gram-positive stains followed the same procedure with one exception: the agglutination test for Staphylococcus aureus on cases not determined. For fungus samples, the first step was doing the macroscopic description followed by the microscopic identification of sexual or asexual structures and its genre.
3 Results
Two hundred samples were taken from the four parks (Table 1), of which 403 isolated found, 197 were bacteria and 206 were fungi.
Table 1 Bacterial and fungal isolations per park and total isolations
| Park | Bacteria | Fungi | Isolations per park |
|---|---|---|---|
| Nacional | 36 | 43 | 79 |
| Timiza | 57 | 53 | 110 |
| El Virrey | 41 | 39 | 80 |
| Simón Bolívar | 63 | 71 | 134 |
| Total Isolations | 197 | 206 | 403 |
3.1 Bacterial isolations from fecal samples
From 50 fecal samples taken, 50 isolations were performed, of which 11 genus and 5 species of bacteria were identified (Table 2, 3).
Table 2 Identification and prevalence of bacteria isolated from all fecal samples
| Genus/specie | Escherichia coli | Citrobacter spp | Enterobacter spp | Aeromonas spp | Edwardsiella spp | Pantoea spp | Klebsiella spp | Plesiomonas spp | Proteus spp | Yersinia spp | Aeromonas hydrophila | Edwardsiella hoshinae | Klebsiella pneumoniae | Salmonella enteritidis |
| Prevalence (%) | 36 | 14 | 12 | 4 | 10 | 8 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
Table 3 Antibiotic susceptibility from fecal samples isolations.
| S(n) | %S | I(n) | %I | R(n) | %R | |
|---|---|---|---|---|---|---|
| Ampicillin | 6 | 12 | 8 | 16 | 36 | 72 |
| Ceftriaxone | 39 | 78 | 4 | 8 | 7 | 14 |
| Ciprofloxacin | 24 | 54.5 | 12 | 27.3 | 8 | 18.2 |
| Trimethoprim | 16 | 42 | 1 | 3 | 21 | 55 |
| Gentamicin | 34 | 89 | 1 | 3 | 3 | 8 |
S=Susceptible, I=Intermediate, R=Resistant, n=number of isolations
3.2 Bacterial isolations from recreational items
From 50 samples taken from recreational items, 76 bacterial isolations were performed, of which 11 genus and 9 species were identified. Isolations of Gram-positive bacilli were reported with a prevalence of 17 % (Tables 4, 5).
Table 4 Identification and prevalence of bacteria isolated from recreational items samples
| Genus/specie | Staphylococcus epidermidis | Staphylococcus aureus | Staphylococcus spp. | Enterobacter spp | Pantoea spp | Streptococcus spp | Yersinia spp | Aeromonas hydrophila | Aeromonas salmonicida | Chryseobacterium idologenes | Klebsiella terrigena | Ochrobactrum antrophi | Salmonella gallinarum | Yersinia enterocolitic |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence (%) | 19.7 | 13.1 | 14.5 | 7.9 | 2.6 | 3.9 | 1.3 | 1.3 | 3.9 | 1.3 | 2.6 | 2.6 | 2.6 | 1.3 |
Table 5 Antibiotic susceptibility of Gram-negative and Gram-positive bacteria from recreational items isolations.
| Gram-negative | S(n) | %S | I(n) | %I | R(n) | %R | Gram-positive | S(n) | %S | I(n) | %I | R(n) | %R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | 16 | 72,7 | 2 | 9,1 | 4 | 18,2 | Oxacillin | 26 | 89,6 | 0 | 0 | 3 | 10,4 |
| Ceftriaxone | 18 | 75 | 3 | 12,5 | 3 | 12,5 | Clindamycin | 26 | 89,6 | 1 | 3,4 | 2 | 7 |
| Ciprofloxacin | 24 | 100 | 0 | 0 | 0 | 0 | Ciprofloxacin | 30 | 93,8 | 1 | 3,1 | 1 | 3,1 |
| Trimethoprim | 20 | 100 | 0 | 0 | 0 | 0 | Trimethoprim | 31 | 96,9 | 0 | 0 | 1 | 3,1 |
| Gentamicin | 20 | 100 | 0 | 0 | 0 | 0 | Gentamicin | 31 | 96,9 | 1 | 3,1 | 0 | 0 |
S=Susceptible, I=Intermediate, R=Resistant, n=number of isolations.
Two Staphylococcus aureus (label as A and B, obtained from El Virrey park) and one isolation of Staphylococcus spp. (obtained from Simón Bolívar park) showed oxacillin resistance. E-test for vancomycin was applied in both S. aureus and one of them showed susceptibility to vancomycin (3 µg/ml) and the other one showed an intermediate pattern (8 µg/ml) (Table 6).
3.3 Isolations from environmental material
3.3.1 Bacterial identification and prevalence
From 50 samples taken from environmental material, 71 bacterial isolates were performed, of which 14 genera and 11 species were identified. Isolations of Gram-positive bacilli were reported with a prevalence of 28% (Tables 7, 8).
Table 7 Identification and prevalence of bacteria isolated from environmental material samples
| Genus/specie | Staphylococcus spp. | Staphylococcus epidermidis | Staphylococcus aureus | Rahnella aquatilis | Aeromonas spp. | Burkholderia spp. | Edwardsiella spp. | Enterobacter spp. | Pantoea spp. | Pasteurella spp. | Streptococcus spp. | Aeromonas salmonicida | Chryseobacterium idologenes | Enterobacter amnigenus | Enterobacter gergoviae | Enterobacter intermedius | Ewingella americana | Pseudomonas aeruginosa | Salmonella gallinarum | Yersinia enterocolitica |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence (%) | 9.8 | 8.4 | 4.2 | 9.8 | 1.4 | 1.4 | 1.4 | 5.6 | 2.8 | 1.4 | 2.8 | 1.4 | 2.8 | 1.4 | 1.4 | 2.8 | 4.2 | 1.4 | 2.8 | 4.2 |
Table 8 Antibiotic susceptibility of Gram-negative and Gram-positive bacteria from environmental material isolations.
| Gram-negative | S(n) | %S | I(n) | %I | R(n) | %R | Gram-positive | S(n) | %S | I(n) | %I | R(n) | %R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | 20 | 74 | 3 | 11 | 4 | 15 | Oxacillin | 13 | 81,2 | 0 | 0 | 3 | 18,8 |
| Ceftriaxone | 24 | 89 | 1 | 3,7 | 2 | 7 | Clindamycin | 13 | 81,2 | 1 | 6,3 | 2 | 12,5 |
| Ciprofloxacin | 23 | 85,2 | 1 | 3,7 | 3 | 11,1 | Ciprofloxacin | 11 | 92 | 0 | 0 | 1 | 8 |
| Trimethoprim | 13 | 72 | 1 | 5,5 | 4 | 22 | Trimethoprim | 15 | 100 | 0 | 0 | 0 | 0 |
| Gentamicin | 18 | 100 | 0 | 0 | 0 | 0 | Gentamicin | 16 | 100 | 0 | 0 | 0 | 0 |
S=Susceptible, I=Intermediate, R=Resistant, n=number of isolations
Likewise, from the same 50 samples taken from environmental material, 112 fungal isolates were performed of which 14 genera were identified and also fungal colonies with sterile mycelium were reported with a prevalence of 7.1%.
3.3.2 Fungal identification and prevalence (Table 9)
Table 9 Identification and prevalence of fungi isolated from environmental material samples
| Genus/specie | Penicillium spp. | Cladosporium spp. | Mucor spp. | Absidia spp. | Alternaria spp. | Bipolaris spp. | Chrysonilia spp. | Epicoccum spp. | Fusarium spp. | Humicola spp. | Nigrospora spp. | Rhizopus spp. | Sordaria spp. | Trichoderma spp. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence (%) | 21.4 | 17.8 | 12.5 | 0.9 | 7.1 | 0.9 | 5.3 | 8.9 | 8.9 | 1.8 | 1.8 | 2.7 | 0.9 | 1.8 |
3.4 Fungal isolations from canine hair
From 50 samples taken from canine hair, 94 isolates were performed, of which 13 genera were identified. Yeast colonies and fungal colonies with sterile mycelium were reported with a prevalence of 8.5% and 4.2% respectively (Table 10).
Table 10 Identification and prevalence of fungi isolated from canine hair samples
| Genus/specie | Penicillium spp. | Cladosporium spp. | Epicoccum spp. | Absidia spp. | Alternaria spp. | Chrysonilia spp. | Cunninghamella spp. | Fusarium spp. | Mucor spp. | Nigrospora spp. | Phoma spp. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence (%) | 38.2 | 22.3 | 9.5 | 2.1 | 2.1 | 3.1 | 1.1 | 1.1 | 5.3 | 1.1 | 1.1 |
4 Discussion
4.1 Bacterial isolation from fecal samples
The high prevalence of Escherichia coli is because this specie is part of the normal intestinal microbiota of mammals like dogs, however most strains of E. coli are non- pathogenic, while those that are pathogenic can cause infections such as travelers diarrhea, abdominal cramps, vomit, urinary tract infections among others 15. Like E. coli, genus Citrobacter spp, Enterobacter spp, Edwardsiella spp, Pantoea spp, Klebsiella, Plesiomonas spp, Proteus spp, Salmonella spp, and Yersinia spp. belong to the Enterobacteriaceae family, within which it has been reported species that normally are part of the intestinal microbiota of some animals or which are isolated from the feces of them 2.
Additionally, a portion of the species belonging to the mentioned genera, such as Klebsiella pneumoniae and Salmonella enteritidis reported here, have medical importance because they generate pathologies in humans related to bacteremia, urinary and lung or respiratory infections, gastroenteritis, meningitis, septicemia and other infections, mainly in people with immunodeficiency 16,17. In the case of S. enteritidis, human infections occur mostly by eating contaminated foods. It is possible to happen the same with a dog, which becomes in the vector of the pathogen and as in this particular case where S. enteritidis was isolated from a feces sample 18.
A few species of Aeromonas spp, can be isolated from some animals and occasionally from human feces, or may also be isolated from freshwater 2. It has been reported that some species of this genus, such as A. hydrophila can cause chronic diarrhea or gastroenteritis, mainly in infants, from enterotoxin production 19.
4.2 Bacterial isolations from recreational and environmental material
Recreational items are constantly manipulated by people who are in contact with other elements of the area or with organic materials such as fallen leaves, plants, water, including soil, in which microorganisms form biofilms of one or more species, like Staphylococcus epidermidis and S. aureus, which can explain the prevalence presented 15. In this situation, both species showed the highest prevalence in both type of samples, recreational items and environmental material. S. aureus (see Figure 1) and S. epidermidis can be found normally in the human skin, however S. aureus is found in a lesser proportion, and both S. aureus and S. epidermidis are species of high clinical importance, because these can cause nosocomial infections, skin infections with a variable severity and in some cases infections by ingestion of contaminated food, being more vulnerable people who have immunodeficiency 2,20. In addition, the strain of S. aureus (B) reported, isolated from El Virrey Park and which showed an intermediate pattern to vancomycin (VISA), have a high clinical importance because these strains represent a considerable threat as potential pathogens that can cause difficulties during treatment 21.
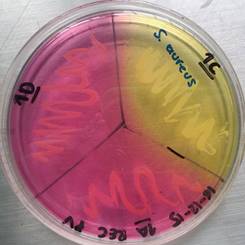
Figure 1 Mannitol salt agar test for Staphylococcus spp. obtained from a sample from El Virrey Park. Author: Camila Andrea Camacho
Moreover the species Rahnella aquatilis showed the same prevalence percentage as the genus Staphylococcus spp. in the environmental samples, possibly because R. aquatilis is isolated from soil, freshwater and in some cases from small invertebrates 22,23. However, it has been reported unusual cases of bacteremia, sepsis and other infections produced by R. aquatilis in wounded patients, elderly or immunocompromised 23.
The bacterial genera and species and its prevalence reported here, can be related with the contact not only people have but also other organisms such as birds, rodents, dogs, etc. with the recreational items and organic sources or material nearby which are full or covered with microorganisms as the biofilms mentioned before (24). For example, Salmonella gallinarum is a species that is mainly spread through birds and can cause gastrointestinal infections 25. Also, it is important to point out the genus Streptococcus spp. which has saprophytic species that are normally isolated from skin, mouth and respiratory tract of humans. Yet, some species are of clinical importance as they can produce diseases like pneumonia, meningitis, tonsillitis and other infections 2,26. Other species reported are potential pathogens because they can produce many infections by its ingestion (in the case of Yersinia enterocolitica) or can also be considered as opportunistic microorganisms, such as Ochrobactrum anthrophi, although its pathogenicity is uncommon and is mainly related to nosocomial infections and immunocompromised patients 27,28.
The antimicrobial susceptibility test results of all isolates showed a considerable high percentage of resistance to ampicillin (44.4%) and, although not as high, trimethoprim (21.1%), being ampicillin only used in the Gram-negative bacilli isolations and trimethoprim in both Gram-negative bacilli and Gram-positive cocci. The high percentage of resistance to both antibiotics can be seen specifically in the feces samples results, where isolations reach the 72% and 55% ampicillin and trimethoprim resistance, which can be associated to the uncontrolled supply of antibiotics to dogs and possible exposure to resistant bacteria in places such as veterinary clinics, where there have been reported a high percentage of multi-resistant strains of potentially pathogenic bacteria that can infect dogs 2,29.
4.3 Fungal isolations from environmental material and canine hair
In both type of samples, the genus Penicillium spp. showed the highest prevalence, probably because is a cosmopolitan genus and it is isolated from the environment, mainly from the soil, thus generating the prevalence result in both environmental material samples and canine hair samples (24). Few cases have been reported where some species of Penicillium spp. cause infection or disease in humans, where the reported ones have generally been in immunocompromised patients 24,30
The genus Cladosporium spp. can be found widely distributed in the environment (like Penicillium spp,) and some species can produce diseases like black tinea, onychomycosis, phaeohyphomycosis, among other, in healthy people 31. Other genera that can produce “feohifomicosis” in healthy and immunocompromised patients are Alternaria spp, (see Figure 2) Bipolaris spp, Phoma spp. and Nigrospora spp. 31,32. Also, some of the species of the genus Fusarium spp. (see Figure 3) and Trichoderma spp, can cause hyalohyphomycosis 32.
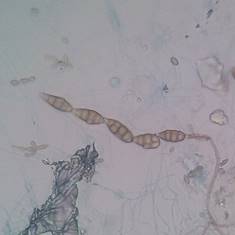
Figure 2 Conidia from Alternaria spp. obtained from an environmental sample, observed through a light microscope with 100x. Author: Diego Gonzalez Lozano
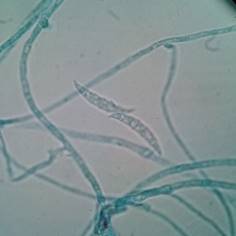
Figure 3 Characteristic septate conidia from Fusarium spp. observed through a light microscope with 100x. Author: Diego Gonzalez Lozano
The genus Mucor spp, had a high prevalence compared to the other fungi genera in samples of environmental material, because it is a genus that is distributed in soil and decaying organic material 31. Mucor spp, as Rhizopus spp. (see Figure 4) and Absidia spp, can cause infections or mucormycosis, affecting eyes and respiratory structures 31,33. Additionally, it has been reported the genus Chrysonilia spp, with a relevant prevalence, where it is important to point out a case of occupational asthma reported in Paris caused by a constant exposure to C. sitophila in a healthy grown man without relevant medical history related to respiratory diseases 34.

Figure 4 Asexual structure of Rhizopus spp. and coenocyte hypha with rhizoids observed through a light microscope with 100x. Author: Diego Gonzalez Lozano
A large proportion of fungal isolates from environmental material are normally found distributed in the environment, carrying out the decomposition of organic matter, and in some cases they don’t represent a threat to human health, except in people with underlying disorders that impair their immune response which makes them vulnerable to infections by opportunistic microorganisms 31. In the canine hair samples we reported mainly environmental genera without relevant dermatophyte genus being identified, probably because the samples were taken from a long part of hair or from the outermost part of the dog’s hair, in which fungi found in the environment can adhere as the dog have contact with the soil, plant material, water, etc. bearing in mind that the results of fungal identification in both types of samples are similar.
5 Conclusion
According to data reported in this article, public parks are areas where several microorganisms can be found, such as fungi and bacteria, which may be pathogens or potential pathogens spread all over the place and some of those can be deposited by animals or by people themselves. It is necessary to have a correct handling of dog feces in these areas because medical importance microorganisms such as Escherichia coli or Salmonella enteritidis can be found, which are capable of causing infections specially with the increasing resistance strains, leading to failure of medical treatment.
It is important to mention that the recreational items and the environmental material of the parks are sources of microorganism’s infections, often opportunistic ones like Staphylococcus aureus, Staphylococcus epidermidis, Enterobacter spp, Rahnella aquatilis, Mucor spp, Cladosporium spp, and other listed here. In consequence, it is essential to have a plan of personal hygiene after having contact with any of these elements or after attending to a park, because many of the reported microorganisms can cause public health problems and can affect a broad range of people.
As we can observe in Tables 11,12, 13, there are an important prevalence of many bacterial and fungal isolates, some of them with real or potential pathogenicity to humans, besides the resistance to antibiotics used in therapy undoubtedly could be revealed a real risk in case of acquisition particularly for inmunocompromised people.
Table 11 Antibiotic susceptibility from all isolations.
| S(n) | %S | I(n) | %I | R(n) | %R | |
|---|---|---|---|---|---|---|
| Ampicillin | 42 | 42.4 | 13 | 13.1 | 44 | 44.4 |
| Ceftriaxone | 81 | 80.2 | 8 | 7.92 | 12 | 11.9 |
| Ciprofloxacin | 112 | 77.8 | 16 | 11.1 | 16 | 11.1 |
| Trimethoprim | 95 | 77.2 | 2 | 1.63 | 26 | 21.1 |
| Gentamicin | 119 | 96.7 | 1 | 0.81 | 3 | 2.44 |
| Oxacillin | 42 | 87.5 | 0 | 0 | 6 | 12.5 |
| Clindamycin | 42 | 87.5 | 2 | 4.17 | 4 | 8.33 |
S=Susceptible, I=Intermediate, R=resistant
Table 12 Total bacterial identification and prevalence
| Genus/specie | Prevalence % |
|---|---|
| Aeromonas hydrophila | 1.0 |
| Aeromonas salmonicida | 2.0 |
| Aeromonas spp. | 1.5 |
| Burkholderia spp. | 0.5 |
| Chryseobacterium idologenes | 1.5 |
| Citrobacter spp. | 3.6 |
| Edwardsiella hoshinae | 0.5 |
| Edwardsiella spp. | 3.0 |
| Enterobacter amnigenus | 0.5 |
| Enterobacter gergoviae | 0.5 |
| Enterobacter intermedius | 1.0 |
| Enterobacter spp. | 8.1 |
| Escherichia coli | 9.1 |
| Ewingella americana | 1.5 |
| Klebsiella pneumoniae | 0.5 |
| Klebsiella spp. | 0.5 |
| Klebsiella terrigena | 1.0 |
| Ochrobactrum antrophi | 1.0 |
| Pantoea spp. | 4.1 |
| Pasteurella spp. | 0.5 |
| Plesiomonas spp. | 0.5 |
| Proteus spp. | 0.5 |
| Pseudomonas aeruginosa | 0.5 |
| Rahnella aquatilis | 5.1 |
| Salmonella enteritidis | 0.5 |
| Salmonella gallinarum | 2.0 |
| Staphylococcus aureus | 6.6 |
| Staphylococcus epidermidis | 10.7 |
| Staphylococcus spp. | 9.1 |
| Streptococcus spp. | 2.5 |
| Yersinia enterocolitica | 2.0 |
| Yersinia spp. | 1.0 |
| Gram-positive bacilli | 16.8 |
Table 13 Total fungal identification and prevalence
| Genus/specie | Prevalence % |
|---|---|
| Absidia spp. | 1.5 |
| Alternaria spp. | 4.9 |
| Bipolaris spp. | 0.5 |
| Chrysonilia spp. | 4.4 |
| Cladosporium spp. | 20.4 |
| Epicoccum spp. | 9.2 |
| Fusarium spp. | 5.3 |
| Humicola spp. | 1.0 |
| Mucor spp. | 9.2 |
| Nigrospora spp. | 1.5 |
| Penicillium spp. | 29.1 |
| Phoma spp. | 0.5 |
| Rhizopus spp. | 1.5 |
| Sordaria spp. | 0.5 |
| Trichoderma spp. | 1.0 |
| Yeasts colonies | 3.9 |
| Fungal colonies with sterile mycelium | 5.8 |














