1 Introduction
The body condition (BC) is defined as a multiple level phenomenon 1 constituted by factors such as the nutritional status 2 , 3, the state of health and the degree of physiological wear of an individual 3 - 7. Because this variable is a key feature in evolutionary ecology, the ethology, management, and the species conservation, it has become an important aspect in ornithological research 8, which has led to the emergence in recent years of numerous indices inferred from morphological, physiological or biochemical measurements of traits with effects on the biological effectiveness of individuals, which seek to simplify data collection in the field and reduce their sacrifice 1 , 8 , 9, allowing the animal to renew its daily behavior, an important factor in the long-term study of species.
Most studies on BC in birds are focused on relating this variable with factors such as activity patterns (migration, mating, competition, winter and social behavior), reproduction and moult 10 - 13, considering to a lesser extent the influence of external environmental conditions 1. In this sense, worldwide, slightly more than 20 % of works devoted to BC are focused on evaluating effects of variables such as the temperature 14, the season 15 - 17, the climatic season 18 , 19, the urbanization 20 ) and the habitat fragmentation 21, which have shown to have an effect on the BC of individuals and their populations.
Studying individually the BC of birds can provide useful information when taking decisions that affect the attributes of the habitat or serve to interpret the population dynamics of species and manage them particularly in a changing climate scenario ( 15. However, on a Neotropical scale, works that evaluate the BC in wild birds are few ( i.e .12 , 13 , 18 , 19 , 21 - 23) and research studies that are focused in analyzing how characteristics of the landscape, the geographic variation or the climatic season affect this variable are scarce 16 , 18 , 19 , 24 , 25. In this context, the aim of our study was to analyze the BC of 13 bird species in two highly intervened landscapes in the tropical dry forest life zone, located in the north of Tolima and the southwest of Huila in the upper Magdalena Valley region.
2 Materials and Methods
2.1 Study Area
The study was done at the Northern Regional University Center (NRUC, Landscape 1), located in the municipality of Armero-Guayabal, Tolima (05°00’38.9” North; 74°54’47.1” West; 275 to 550 m) and in the localities El Tabor (ET: El Pedernal rural settlement, 02°18’50.8” North, 75°40’08.8” West, 810 meters above sea level) and La Ensillada (LE: Domingo Arias rural settlement, 02°22’57.2” North; 75°37’47.7” West; 1081 m) (ET-LE; Landscape 2) located in the municipalities of El Agrado and Paicol, Huila, during years 2012 and 2013.
Landscapes are separated from each other in distance by 310 km approximately. They are located in the upper Magdalena Valley and have characteristics of the tropical dry forest life zone. Due to the fertility of its soils, this area has been a point of development of human populations and an object of transformation. Therefore, both landscapes mosaics are dominated by agricultural and livestock areas (crops, pastures and bare soils), finding lesser natural coverages (remnants of forest with relictual conditions, secondary forests, bodies of water, rocky outcrops, among others) and human infra-structure 26 , 27.
The Huila landscape (ET-LE: average rainfall 1,791 mm, average temperature 28° C) has vegetation cover such as secondary forests, gallery forests, scrubs and grasslands, which are dominated by species of the families Fabaceae, Poaceae and Euphorbiaceae 28. Meanwhile, the Tolima landscape (NRUC: average rainfall 900-1,800 mm, average temperature 26° C-28° C) has coverages such as secondary forests, gallery forests, scrubs, living fences, crops and pastures, where the dominant plants species belong to the families Poacea, Meliaceae, ZygophyIlaceae, and Fabaceae 29 , 30.
2.2 Data Collection
During the field phase, monthly samplings were made using 10 mist nets (2.5 m x 12 m; 36 mm mesh) which in the NRUC were operated for two days and one morning per month during six months (2012: January, February, May, July and December; 2013: March) from 06:00 h -11:00 h and 15:00 h -17:30 h (total sampling effort: 1,200 h-net) within the Bird Monitoring Program developed by the Zoology Research Group of the University of Tolima, and in ET-LE mist-nets were operated for seven days per month during 12 months (February-December 2012; January 2013) from 06:00-11:00 (total sampling effort: 4,200 h-net). The captured individuals were determined up to the specie level, and their body mass information (g), wing chord length (mm) and tarsal length (mm) were recorded in field formats (measured through. A 500 g digital balance ± 0.1 g precision, a 150 mm stopped rule and a 150 mm dial caliper ± 0.1 mm precision respectively, were used).
The selection of species to be evaluated was made taking into account their resident status, their capture in at least four months of sampling, the total number of records obtained (n≥5) and the registration of individuals in both climatic seasons (dry-rainy, n≥2 for each season); obtaining a sample size of 457 captures (245 in NRUC and 212 in ET-LE) distributed in two orders, six families and 13 species with five to 43 individuals (Table 1) was obtained.
Table 1 Number of captures (n) per landscape, selected character and description of model M= aLb in 13 bird species in the tropical dry forest region of the upper Magdalena Valley during years 2012 and 2013 (NRUC: Northern Regional University Center, Tolima; ET-LE: El Tabor and La Ensillada, Huila).
| Species | n NRUC | n ET-LE | Character best model (mm) | Model NRUC | Model ET-LE |
|---|---|---|---|---|---|
| Common Ground-dove | 31 | 30 | Tarsus length | M= 3.16(L)0.07 | M= 3.28(L)0.04 |
| Ruddy Ground-dove | 40 | 9 | Tarsus length | M= 3.64(L)0.02 | M= 7.14(l)-0.61 |
| White-fringed Antwren | 12 | 12 | Tarsus length | M= 1.8(L)0.24 | M= 0.68(L)1.16 |
| Mouse-colored Tyrannulet | 20 | 7 | Tarsus length | M= 7.8(L)0.22 | M= 0.36(L)1.74 |
| White-bearded Manakin | 12 | 5 | Tarsus length | M= 1.78(L)0.38 | M= 0.96(L)0.96 |
| Gray-headed Tanager | 6 | 20 | Tarsus length | M= 3.8(L)-0.1 | M= 3.3(L)0.02 |
| Crimson-backed Tanager | 8 | 11 | Wing chord length | M= 1.87(L)0.38 | M= 3.34(L)-0.01 |
| Streaked Saltator | 16 | 21 | Tarsus length | M= 1.54(L)0.38 | M= 0.94(L)0.87 |
| Blue-black Grassquit | 27 | 23 | Tarsus length | M= 3.52(L)0.02 | M= 1.49 (L)0.8 |
| Ruddy-breasted Seedeater | 36 | 13 | Tarsus length | M= 2.08(L)-0.04 | M= 0.09(L)3.16 |
| Slate-colored Seedeater | 10 | 6 | Wing chord length | M= 0.62(L)1 | M= 0.11(L)2.26 |
| Pileated Finch | 15 | 12 | Tarsus length | M= 2.49(L)0.1 | M= 1.47(L)0.58 |
| Rufous-capped Warbler | 12 | 43 | Wing chord length | M= 0.62(L)0.97 | M= 2.31(L)0.04 |
The order Columbiformes (family Columbidae) was represented by Common Ground-dove (Columbina passerina) and Ruddy Ground-dove (Columbina talpacoti), while the order Passeriformes was represented by White-fringed Antwren (Formicivora grisea) (Thamnophilidae); Mouse-colored Tyrannulet (Phaeomyias murina) (Tyrannidae); White-bearded Manakin (Manacus manacus) (Pipridae); Gray-headed Tanager (Eucometis penicillata), Crimson-backed Tanager (Ramphocelus dimidiatus), Streaked Saltator (Saltator striatipectus), Blue-black Grassquit (Volatinia jacarina), Ruddy-breasted Seedeater (Sporophila minuta), Slate-colored Seedeater (Sporophila schistacea), Pileated Finch (Coryphospingus pileatus) (Thraupidae) and Rufous-capped Warbler (Basileuterus rufifrons) (Parulidae).
2.3 Scaled Mass Index
The BC by species was estimated using the Scaled Mass Index (SMI) proposed 3, which was calculated through the formula
2.4 Data Analysis
The variation in the SMI between landscapes and climatic seasons was determined by the nonparametric method of Kruskall-Wallis once the assumptions of normality (Shapiro-Wilks) and homoscedasticity (Levene's test) were rejected. All analyses were performed with the InfoStat® program 31 using a significance level of 0.05.
3 Results
The species White-fringed Antwren (H= 11.21, N= 24, P= 0.01), Streaked Saltator (H= 10.36, N= 37, P= 0.01, df= 1), Slate-colored Seedeater (H= 4.03, N= 16, P= 0.04, df= 1) and Rufous-capped Warbler (H= 14.06, N= 55, P= 0.01, df= 1) showed significant differences in the SMI between landscapes (Figure 1), while the species Common Ground-dove (H= 0.08, N= 61, P= 0.77, df= 1), Ruddy Ground-dove (H= 3.7, N= 49, P= 0.05, df= 1), Mouse-colored Tyrannulet (H= 0.31, N= 27, P= 0.58, df= 1), White-bearded Manakin (H= 1.6, N= 17, P= 0.23, df= 1), Gray-headed Tanager (H= 0.73, N= 26, P= 0.39, df= 1), Crimson-backed Tanager (H= 0.14, N= 19, P= 0.73, df= 1), Blue-black Grassquit (H= 0.52, N= 50, P= 0.47, df= 1), Ruddy-breasted Seedeater (H= -22.87, N= 49, P > 0.99, df= 1) and Pileated Finch (H= 3, N= 27, P= 0.08, df= 1) didn’t show differences (Table 2).
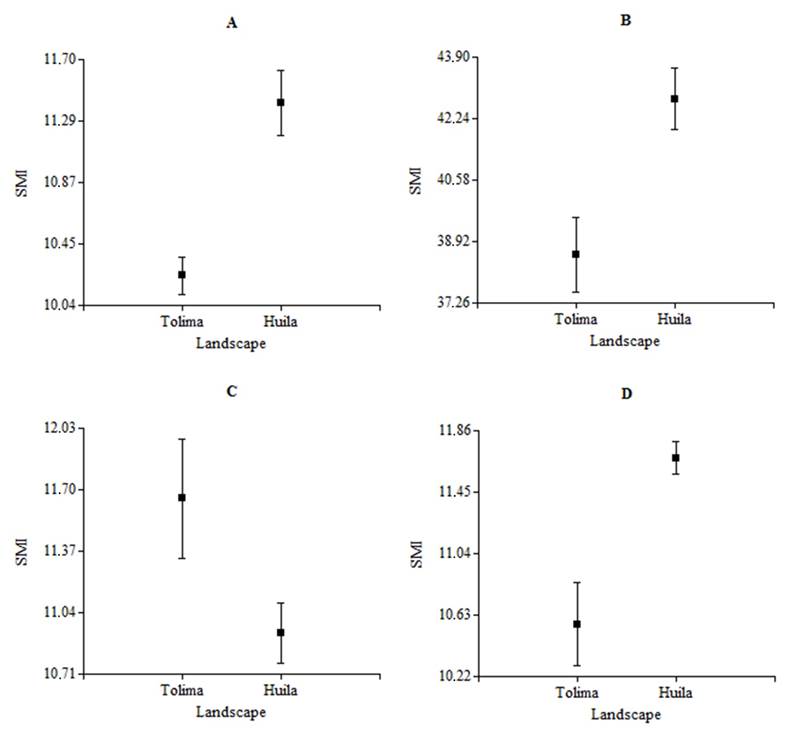
Figure 1 Scaled Mass Index (± SE) of some species of birds between landscapes (NRUC: Northern Regional University Center, Tolima vs ET-LE: El Tabor and La Ensillada, Huila), A) White-fringed Antwren (F. grisea); B) Streaked Saltator (S. striatipectus); C) Slate-colored Seedeater (S. schistacea); D) Rufous-capped Warbler (B. rufifrons) in years 2012 and 2013.
Table 2 Mean values of Scaled Mass Index (± SE) per landscape (NRUC: Northern Regional University Center, Tolima; ET-LE: El Tabor and La Ensillada, Huila), in 13 bird species in the tropical dry forest region of the upper Magdalena Valley during years 2012 and 2013.
| Species | Mean SMI (± SE) NRUC | Mean SMI (± SE) ET-LE | Species | Mean SMI (± SE) NRUC | Mean SMI (± SE) ET-LE |
| Common Ground-dove | 31.88 ± 5.23 | 31.61 ± 3.88 | Streaked Saltator | 38.57 ± 1.01 | 42.76 ± 0.83 |
| Ruddy Ground-dove | 40.49 ± 1.15 | 44.83 ± 1.69 | Blue-black Grassquit | 9.46 ± 0.17 | 9.39 ± 0.2 |
| White-fringed Antwren | 10.24 ± 0.13 | 11.41 ± 0.22 | Ruddy-breasted Seedeater | 7.46 ± 0.15 | 7.59 ± 0.43 |
| Mouse-colored Tyrannulet | 9.78 ± 0.18 | 9.67 ± 0.29 | Slate-colored Seedeater | 11.65 ± 0.32 | 10.93 ± 0.16 |
| White-bearded Manakin | 15.00 ± 0.62 | 15.30 ± 0.22 | Pileated Finch | 15.99 ± 0.22 | 15.44 ± 0.15 |
| Gray-headed Tanager | 30.53 ± 0.82 | 29.93 ± 0.35 | Rufous-capped Warbler | 10.57 ± 0.27 | 11.67 ± 0.11 |
| Crimson-backed Tanager | 26.20 ± 0.57 | 27.09 ± 0.85 |
On the other hand, when comparing the SMI values in the rainy season between landscapes, we find that the species Streaked Saltator (H= 4.04, N= 21, P= 0.04, df= 1) and Rufous-capped Warbler (H= 9.84, N= 30, P= 0.01, df= 1) showed significant differences (Figure 2). However the species Common Ground-dove (H= 0.01, N= 27, P= 0.92, df= 1), Ruddy Ground-dove (H= 0.19, N= 16, P= 0.71, df= 1), White-fringed Antwren (H= 3.75, N= 10, P= 0.06, df= 1), Mouse-colored Tyrannulet (H= 1.13, N= 11, P= 0.35, df= 1), White-bearded Manakin (H= 1.78, N= 8, P= 0.21, df= 1), Gray-headed Tanager (H= 1.04, N= 17, P= 0.33, df= 1), Crimson-backed Tanager (H= 0.72, N= 12, P= 0.44, df= 1), Blue-black Grassquit (H= 0.46, N= 29, P= 0.5, df= 1), Ruddy-breasted Seedeater (H= 2.72, N= 20, P= 0.1, df= 1), Slate-colored Seedeater (H= 0, N= 5, P > 0.99, df= 1) and Pileated Finch (H= 2.78, N= 14, P= 0.1, df= 1) didn’t show such differences.
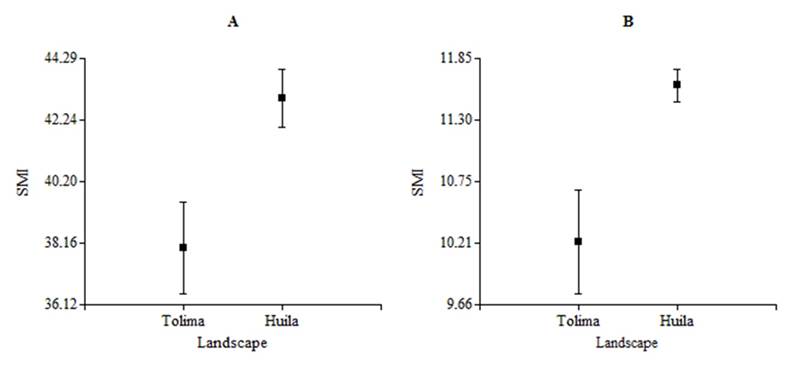
Figure 2 Scaled Mass Index (± SE) of some species of birds between landscapes during the rainy season (NRUC: Regional University Center of the North, Tolima vs ET-LE: El Tabor and La Ensillada, Huila), A) Streaked Saltator (S. striatipectus); B) Rufous-capped Warbler (B. rufifrons) in years 2012 and 2013.
In the same way, when values of SMI are compared in the dry season between landscapes, we find that the species White-fringed Antwren (H= 7.47, N= 14, P= 0.01, df= 1), Slate-colored Seedeater (H= 5.14, N= 11, P= 0.02, df= 1) and Rufous-capped Warbler (H= 5.1, N= 25, P= 0.02, df= 1) showed significant differences (Figure 3), while the species Common Ground-dove (H= 0.31, N= 34, P= 0.58, df= 1), Ruddy Ground-dove (H= 0.39, N= 33, P= 0.53, df= 1), Mouse-colored Tyrannulet (H= 0, N= 16, P > 0.99, df= 1), White-bearded Manakin (H= 0.07, N= 9, P= 0.86, df= 1), Gray-headed Tanager (H= 0, N= 9, P > 0.99, df= 1), Crimson-backed Tanager (H= 0.5, N= 7, P= 0.63, df= 1), Streaked Saltator (H= 2.61, N= 16, P= 0.12, df= 1), Blue-black Grassquit (H= 0.1, N= 21, P= 0.75, df= 1), Ruddy-breasted Seedeater (H= 0.11, N= 29, P= 0.74, df= 1) and Pileated Finch (H= 1.4, N= 13, P= 0.27, df= 1) didn’t show such differences for the variable in question. Ruddy-breasted Seedeater (H= 5.53, N= 36, P= 0.02, df= 1) was the only species that showed significant differences in its SMI between the dry and rainy period only in the Tolima landscape (NRUC), while in the Huila landscape (E-T) none of species showed significant differences in their BC between climatic seasons (Table 3).
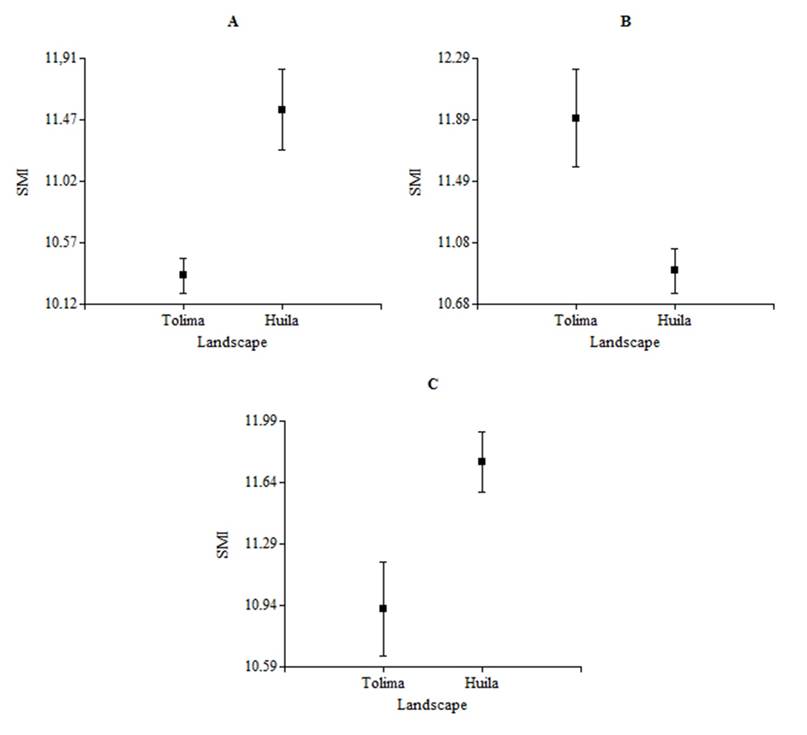
Figure 3 Scaled Mass Index (± SE) of some bird species between landscapes during the dry period (NRUC: Regional University Center of the North, Tolima vs ET-LE: El Tabor and La Ensillada, Huila), A) White-fringed Antwren (F. grisea); B) Slate-colored Seedeater (S. schistacea) y C) Rufous-capped Warbler (B. rufifrons) in years 2012 and 2013.
Table 3 Mean values of the Scaled Mass Index (± SE) and Kruskal-Wallis (K-W) test results of 13 bird species in each climatic season in the tropical dry forest region of the upper Magdalena Valley during years 2012 and 2013 (NRUC: Northern Regional University Center, Tolima vs ET-LE: El Tabor and La Ensillada, Huila).
| Species | Mean SMI NRUC | K-W | Mean SMI ET-LE | K-W | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Dry | n | Rainy | n | Dry | n | Rainy | |||
| Common Ground Dove | 20 | 32.53 ± 1.37 | 11 | 30.7 ± 0.88 | H= 0.49 P= 0.48 | 14 | 31.43 ± 0.97 | 16 | 31.76 ± 1.05 | H= 3.9x10-3 P= 0.95 |
| Ruddy Ground Dove | 30 | 38.80 ± 1.1 | 10 | 45.57 ± 2.77 | H= 2.2 P= 0.14 | 3 | 41.13 ± 2.03 | 6 | 46.68 ± 1.99 | H= 2.4 P= 0.17 |
| White-fringed Antwren | 9 | 10.33 ± 0.13 | 3 | 9.97 ± 0.33 | H= 0.08 P= 0.36 | 5 | 11.54 ± 0.29 | 7 | 11.31 ± 0.32 | H= 2.91 P= 0.8131 |
| Mouse-colored Tyrannulet | 11 | 9.62 ± 0.26 | 9 | 9.97 ± 0.27 | H= 0.98 P= 0.32 | 5 | 9.80 ± 0.40 | 2 | 9.35 ± 0.05 | H= 2.4 P= 0.86 |
| White-bearded Manakin | 6 | 16.07 ± 1.01 | 6 | 13.93 ± 0.45 | H= 0.41 P= 0.12 | 3 | 15.37 ± 0.27 | 2 | 15.2 ± 0.5 | H= 0.75 P= 0.7 |
| Gray-headed Tanager | 2 | 30.15 ± 2.45 | 4 | 30.73 ± 0.8 | H= 0.21 P= 0.8 | 7 | 30.04 ± 0.62 | 13 | 29.86 ± 0.44 | H= 0.04 P= 0.84 |
| Crimson-backed Tanager | 4 | 26.7 ± 0.23 | 4 | 25.7 ± 1.14 | H= 3 P= 0.26 | 3 | 26.03 ± 0.96 | 8 | 27.49 ± 1.12 | H= 2.67 P= 0.4 |
| Streaked Saltator | 13 | 38.7 ± 1.21 | 3 | 38.00 ± 1.51 | H= 4.5x10-3 P= 0.9 | 3 | 41.63 ± 0.44 | 18 | 42.95 ± 0.97 | H= 0.06 P= 0.8 |
| Blue-black Grassquit | 12 | 9.68 ± 0.25 | 15 | 9.27 ± 0.23 | H= 0.77 P= 0.38 | 9 | 9.63 ± 0.38 | 14 | 9.24 ± 0.22 | H= 0.72 P= 0.39 |
| Ruddy-breasted Seedeater | 22 | 7.23 ± 0.13 | 14 | 7.84 ± 0.31 | H= 5.53 P= 0.02 | 7 | 7.97 ± 0.56 | 6 | 7.15 ± 0.66 | H= 0.73 P= 0.42 |
| Slate-colored Seedeater | 7 | 11.9 ± 0.32 | 3 | 11.07 ± 0.73 | H= 0.01 P= 0.48 | 4 | 10.90 ± 0.15 | 2 | 11.00 ± 0.5 | H= 0.86 P > 0.99 |
| Pileated Finch | 10 | 15.84 ± 0.22 | 5 | 16.3 ± 0.52 | H= 1.5 P= 0.24 | 3 | 15.23 ± 0.41 | 9 | 15.51 ± 0.16 | H= 0.31 P= 0.48 |
| Rufous-capped Warbler | 6 | 10.92 ± 0.27 | 6 | 10.22 ± 0.46 | H= 3.69 P= 0.38 | 19 | 11.75 ± 0.17 | 24 | 11.61 ± 0.14 | H= 0.37 P= 0.54 |
4 Discussion
The absence of significant differences in the BC between landscapes in nine of the evaluated species suggests that in a large part of the resident bird populations of tropical dry forest of the upper Magdalena Valley this variable behaves similarly in this region of Colombia. However, in four species it is evident that their individuals are affected by characteristics of each landscape, so the highest values in the Slate-colored Seedeater’s SMI in the Tolima landscape can be related to its trophic guild, since this species feeds mainly on seeds of Poaceaes 32, which are highly dominant within the NRUC. On the other hand, White-fringed Antwren, Streaked Saltator and Rufous-capped Warbler showed the highest SMI values in the Huila landscape; being mainly insectivorous or frugivorous. These species BC is favored in less mature habitats and with greater vertical vegetation heterogeneity -as the case of ET-LE-, since these provide a greater abundance and diversity of insects 33, and their production of fruits -favored by high levels of light that reach the undergrowth thanks to its open canopy- can be higher and more constant compared to slightly more mature habitats 34.
This result is similar to that reported by 25, who despite finding significant differences in the BC of two populations of Chestnut-backed Antbird (Poliocrania exsul) in agricultural landscapes of southwestern Costa Rica (Los Cusingos and Boruca), registered that in general, populations of this species don’t present BC values different from others located in fragmented landscapes on the Atlantic side of the country, suggesting that Chestnut-backed Antbird doesn’t affect its BC due to landscape conditions (fragmentation).
On the other hand, the assessment of the SMI between landscapes for each climatic season showed that the lack of significant differences in nine of the species could be explained by the hypothesis that a poor quality habitat doesn’t restrict individuals when climatic conditions are optimal 35 and almost stable 19, or that in these species the percentage of dominant birds (males and adults) and subordinates (females and juveniles) doesn’t vary significantly throughout the year, so success in obtaining sufficient resources to maintain the BC and persist through the climatic periods will be constant 24. However, this does not apply to the community in general, since four of the species present significant differences in their BC between landscapes in such a way that during the rainy period Streaked Saltator and Rufous-capped Warbler showed the highest values in the Huila landscape, while during the dry period White-fringed Antwren and Rufous-capped Warbler Warbler showed the highest values in the Huila landscape and Slate-colored Seedeater in the Tolima landscape.
In addition, the absence of significant differences in the SMI when its changes are evaluated in both climatic season within each landscape suggests that variation in climatic and environmental factors (linked to changes in precipitation) in the tropical dry forest of the upper Magdalena Valley does not affect the BC of the species, which is similar to that obtained by 36 ) who found that individuals of the Common Reed Bunting (Emberiza schoeniclus) in Poland do not present significant seasonal differences in BC.
In our study, the only exception to this pattern was the Ruddy-breasted Seedeater, in which the SMI showed significant differences between climatic periods in the Tolima landscape, presenting the highest values during the rainy season. This could be due to the fact that rains are essential for many Passeriformes, because during this period there is a greater abundance of insects and their larvae 37 - 40, which are an important source of food even for non-insectivorous species 41 such as the species in question, which feeds mainly on seeds of plants of the Poaceae and Cyperaceae families but sporadically consumes Hymenoptera and Orthoptera insects 42. In addition, this result is similar to that reported by (Angelier F, Tonra CM, Holberton RL, Marra PP, 2011) 35 who found that in the American Redstart (Setophaga ruticilla), the physical condition presents the highest values at the time with the highest rainfall.
We conclude that comparing distant landscapes with similar abiotic characteristics, some species can present significant differences in their BC, which can be given by specific factors of each habitat. In addition, we show how the climatic season can affect the BC of some bird species present in the tropical dry forest of the upper Magdalena Valley but does not significantly influence the BC of the community in general. We recommend making studies in the Neotropics relating BC with variables such as vegetation structure, inter and intraspecific competition and food availability.















