Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Odontología Universidad de Antioquia
Print version ISSN 0121-246X
Rev Fac Odontol Univ Antioq vol.23 no.1 Medellín July/Dec. 2011
ARTÍCULOS ORIGINALES DERIVADOS DE INVESTIGACIÓN
Adjunctive systemic administration of moxifloxacin versus ciprofloxacin plus metronidazole in the treatment of chronic periodontitis harboring gram-negative enteric rods: I microbiological and clinical effects1
Isabel Cristina Guzmán2; Hugo Grisales Romero3; Carlos Martín Ardila Medina4
1 This study was sponsored in part by a grant from the National
Public Health School and the Epidemiology Group of the
Universidad de Antioquia. The authors report no conflict of interest
related to this study
2 Periodontist, Assistant Professor, School of Dentistry, Universidad
de Antioquia, Colombia
3 Ph.D. in Epidemiology, Titular Professor National Public Health
School Universidad de Antioquia, Colombia
4 Periodontist, Ph. D. Associate Professor School of Dentistry
Universidad de Antioquia, Colombia. Director, Group of Biomedical
Stomatology, Universidad de Antioquia, Medellín, Colombia
Key Words: antimicrobial(s), microbiology, periodontitis, Gram-negative enteric rods.
SUBMITTED: MARCH 11/2011 - ACCEPTED: SEPTEMBER 13/2011
CORRESPONDENCECarlos M. Ardila
Calle 64 N.° 52-59
Medellín, Colombia
Fax: (57-4) 219 67 60
e-mail: martinardila@gmail.com
Guzmán IC, Grisales H, Ardila CM. Adjunctive systemic administration of moxifloxacin versus ciprofloxacin plus metronidazole in the treatment of chronic periodontitis harboring gram-negative enteric rods: I microbiological and clinical effects. Rev Fac Odontol Univ Antioq 2011; 23(1): 92-110.
ABSTRACT
INTRODUCTION: preliminary clinical findings indicate that periodontal lesions associated with Gram-negative enteric rods do
not respond to conventional treatment modalities. The aim of the present study was to evaluate and compare the clinical and microbiological
effects of scaling and root planing (SRP) combined with systemic administration of moxifloxacin (MOX) or ciprofloxacin plus metronidazole
(CIPRO + MET) in the treatment of chronic periodontitis.
METHODS: seventy-six patients participated in this randomized clinical trial,
and they were divided into two groups. Subjects were treated with SRP plus adjunctive MOX (MOX group; n = 38) or SRP plus adjunctive CIPRO + MET (CIPRO + MET GROUP; n = 38). Clinical and microbiological data were recorded at baseline and at 3 and 6 months
after treatment. The significant changes in clinical and microbiological parameters between and within the groups were measured using the
Mann-Whitney test and Wilcoxon’s rank test respectively.
RESULTS: after six months, both treatment groups showed a significant reduction
in probing depth and bleeding on probing (P < 0.05) and better clinical attachment. A statistically significant reduction of the proportion
of sites > 6 mm was also observed. Gram-negative enteric rods and Aggregatibacter actinomycetemcomitans were not identified in either group six months after baseline.
CONCLUSIONS: this study provides evidence of the benefit of using MOX or CIPRO+MET as adjunct to SRP
in patients with chronic periodontitis harboring Gram-negative enteric rods. However, MOX may be the antibiotic of choice in view of its
few adverse effects and single dose treatment per day.
Palabras clave: antimicrobial(s), microbiology, periodontitis, Gram-negative enteric rods.
INTRODUCCIÓN
It has been commonly concluded that scaling and root planing (SRP) is an effective treatment approach; however, this procedure does not frequently lead to the microbiological changes necessary for maintaining the long-term stability of the clinical benefits initially achieved.1
The adjunctive use of systemically administered antibiotics has been shown to provide a better clinical outcome, particularly in terms of probing depth (PD) reduction and attachment-level gain than SRP in subjects with chronic periodontitis.2, 3 Periodontal pathogens such as Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola are more effectively reduced by the use of systemic antibiotics in chronic periodontitis.1, 4-6 Furthermore, adjunctive antimicrobial therapy with systemic antibiotics affects periodontal pathogens residing in non-periodontal mucosal surfaces.7
On the other hand, Gram-negative enteric rods (GNER) are opportunistic pathogens in a wide range of human infections8 and they can be detected in the subgingival environment of periodontitis subjects. 9-20 These microorganisms are able to produce virulence factors and have shown the capacity to invade human tissue.11 GNER are capable of degrading elastin and have been shown to produce hydrolytic enzymes that might accord with proteolytic activities expressed by recognized periodontopathogens, during destruction of tooth-supporting tissues.11 The isolation from periodontal sites of elastin degrading Klebsiella pneumonia strain, a usual GNER that may cause pneumonia, emphasizes that the oral cavity is a reservoir of pathogens that may spread and cause severe infections in the lower respiratory tract.11
Lecithinase production by GNER strains from periodontal lesions may disrupt transduction pathways in endothelial cells, platelets and neutrophils leading to uncontrolled production of intracellular mediators and adhesion molecules, thus altering the traffic of granulocytes to the infected tissue.11
Subgingival GNER often persist after periodontal debridement and surgery and have been implicated as key pathogens in cases of refractory periodontitis. 10, 12-15 These microorganisms were detected at higher frequency and in higher proportions in patients with failing implants.13 Additionally, they show less susceptibility to chlorhexidine16 and the fact that these microorganisms exhibit in vitro resistance to the majority of adjunctive antibiotics used to treat periodontitis,17-20 means that periodontal lesions associated with these organisms do not respond to conventional treatment modalities.10 Ciprofloxacin therapy has the potential to eradicate GNER from periodontal pockets but has little activity against the main putative periodontopathogens,10, 21 therefore, it should be combined with an antimicrobial agent active against anaerobes as metronidazole.9, 10 Conversely, in earlier papers, moxifloxacin (MOX) has shown in vitro activity against GNER,20 and in vitro22 and in vivo4 efficacy against periodontopathogens. These articles also outlined a procedure for clinical trials to investigate the effects of MOX in the treatment of patients with chronic periodontitis harboring GNER in subgingival plaque.
To our knowledge no studies have assessed the in vivo efficacy and clinical applicability of MOX against GNER present in subgingival plaque. Therefore, the aim of the present study was to evaluate and compare the clinical and microbiological effects of SRP combined with systemic MOX or ciprofloxacin plus metronidazole (CIPRO + MET) in the treatment of subjects with chronic periodontitis.
MATERIALS AND Y METHODS
Subjects
Seventy-six systemically healthy subjects (45 women and 31 men), aged 27 to 66 years who attended the dental clinics of Universidad de Antioquia were recruited from October 2008 to March 2009. Informed and written consent was obtained from each participant.
The study design was approved by the Ethics Committee on Human Research of the University Investigation Department of the Universidad de Antioquia according to the Declaration of Helsinki on experimentation involving human subjects. Patients with a diagnosis of chronic periodontitis were considered candidates for the study. Subjects were > 26 years of age, had at least 20 natural teeth, including at least 1 molar tooth in each quadrant, and at least eight sites with PD = 5 mm. Exclusion criteria included diabetes, cardiovascular disease, or any other systemic disease that could alter the course of periodontal disease. Pregnant or nursing women, consumption of systemic antimicrobials or anti-inflammatory drugs in the last six months, and periodontal therapy during the last six months also served as exclusion criteria.
Experimental design and treatment
This was a parallel arm, randomized trial with masking of examiner, clinician performing treatment and statistician, with 6 months of follow-up. Subjects were randomly assigned by a computer-generated table to receive one of the two treatments. The assignment of subjects to the treatment groups was carried out by the clinic coordinator remote from the study. The randomization code was held centrally by the clinic coordinator and was not broken until completion of the data analyses. The two treatment groups consisted of SRP combined with systemically administered MOX at the dosage of 400 mg once daily for 7 days (MOX group) or SRP combined with systemically administered CIPRO+MET at the dosage of 1g once daily plus 500 mg b.i.d. for 7 days (CIPRO + MET group). One-stage fullmouth SRP under local anesthesia was performed in approximately two hours and half by the same experienced periodontist. The endpoint of SRP was a tactile smooth root surface. The adjunctive agents were started at the SRP visit. Subjects in the MOX and CIPRO + MET groups were extensively informed about the intake of the prescribed medication.
Subjects were clinically and microbiologically monitored at baseline (before therapy) and at 3 and 6 months post-therapy. During the monitored sessions, oral hygiene was evaluated and home care instructions were re-emphasized. All subjects came for recall visits and received oral hygiene evaluations.
The recall visits were made on a 2-week interval during the 6 months after treatment.
Compliance
A dental assistant called each subject during the next 6 days by telephone to remind him/her to take the remaining doses. The same dental assistant, not involved in the randomization process, recorded compliance with medication intake and occurrence of adverse events. The subjects were asked to bring the boxes containing the medication the week after the SRP visit, when the pills were counted in order to check any inaccuracy in drug taking.
Clinical evaluation
Medical history was taken and clinical and radiographic examinations were conducted for each patient. At each monitoring visit, visible plaque (0/1), bleeding on probing (BOP) (0/1), probing depth (PD) (in mm) and clinical attachment level (CAL) (in mm) were measured at six sites per tooth (mesiobuccal, buccal, distobuccal, distolingual, lingual and mesiolingual) in all teeth excluding third molars. The PD and CAL measurements were recorded to the nearest millimeter by a calibrated standard probe (UNC-15, Hu-Friedy, Chicago, IL). Measurements at all visits for a given subject were made by the same blinded, trained and calibrated clinician. The clinician making the clinical measurements did not perform the therapy on the subjects. The intra-examiner reproducibility was assessed before and during the experimental period according to the method described by Araujo et al.23 Repeated measurements were performed on a total of 10 periodontal patients (who were not participating in this study), five of whom were examined immediately before the clinical trial, and the other five during the experimental period. Duplicate measurements were conducted in groups of two patients with at least 2 h between each examination. The intra-class correlation coefficients for mean PD and CAL were 0.92 and 0.91, respectively. The examiner’s reproducibility of measurements made before and during the study was similar. The diagnosis of chronic periodontitis was made based on criteria defined at the workshop sponsored by the American Academy of Periodontology (AAP).24
Microbial sampling
Microbial sampling on periodontitis patients was performed on pockets = 5 mm. The deepest six pockets were selected for sampling. After removing supragingival plaque with curets and isolating the area with cotton pellets, the paper points (Maillefer, Ballaigues, Switzerland) were inserted into each periodontal pocket for 20 seconds. The paper points were transferred to a tube with Viability Medium Göteborg Anaerobically (VGMA) III medium.25 The samples were analyzed using microbial culture techniques for the presence of periodontopathic bacteria according to Slots.26 All samples were processed in = 24 hours at room temperature and immediately incubated in CO2 and anaerobic culture systems. Brucella blood agar medium was incubated at 35 °C in an anaerobic jar for 7 days. The trypticase-soy with serum, bacitracin, and vancomycin medium was incubated in 10% CO2 in air at 37 °C for 4 days. Presumptive identification was performed according to the methods described26, 27 and using a commercial identification micromethod system (RapID ANA, Remel, Norcross, GA) for A. actinomycetemcomitans, P. gingivalis and T. forsythia. Total viable counts (TVC) were defined as the total number of colony-forming units obtained on non-selective media plates. Species found on selective media were enumerated and their percentage of TVC was calculated.
Isolation of GNER by culture. After placement for 20 s, the paper points were pooled into a vial containing 2.0 ml of VMGA III transport medium.25 The sample vials were maintained at room temperature, transferred to the laboratory and processed within 4 h after sampling. After the vials were placed in an incubator for 30 min at 37 °C, bacterial plaque was mechanically dispersed with a test tube mixer at the maximal setting for 60 s. Serial 10-fold dilutions were prepared in pepton water, and aliquots were plated on MacConkey agar. The plates were incubated aerobically at 37°C for 24 h. Each isolate was characterized according to colonial and cellular morphology and Gram-stain characteristics. GNER were speciated using a standardized biochemical test (BD, Sparks, MD). TVC were defined as the total number of colony-forming units obtained on non-selective media plates. Species found on selective media were enumerated and their percentage of TVC was calculated.
Each patient provided a pooled subgingival plaque sample. Equal numbers of isolates were used from each subject.
Statistical Analysis
Data were entered into an Excel (Microsoft office 2007) database and were proofed for entry errors. The subject was the unit for the basic statistical analysis. Mean values ± SD and the proportions of sites within various categories of scoring units were calculated for data description. Normal distribution of continuous variables was verified with the Kolmogorov-Smirnov test. Categorical data were analysed with the X2 test, and the percentage data between the two groups were compared with the Mann-Whitney test. Differences between groups and between different timepoints within groups were tested by the Mann-Whitney test and the Wilcoxon’s rank test, respectively.
Only sites with initial PD = 4 mm were included in the statistical analyses, as shallower sites did not receive SRP, although they were treated with subgingival scaling. PD was set as the primary outcome and CAL as secondary outcome of the present study. Explanatory variables that were recorded were BOP and presence of enteric rods and periodontopathogens tested.
Data concerning sites with PD > 6 mm were analysed separately with the site as the observational unit. At each timepoint, for each group, the proportion of sites > 6 mm was calculated and changes of these proportions from baseline between groups were compared using the Mann-Whitney test.
Microbiological data were analysed with the subject as the observational unit. The number of subjects positive for the investigated species at any of the sampled sites were estimated at all timepoints. In order to identify specific differences between pairs of groups at each timepoint the Mann-Whitney test was applied, while differences between timepoints within each group were tested with the Wilcoxon’s test.
The significance level was set at 0.05 for all tests.
All data handling and statistical testing were performed with a software package (SPSS, Statistical Package for the Social Sciences, version 15, Chicago, IL).
Sample size calculation
Study sample size calculations were based on subject level analyses as the study randomized individuals. The ideal sample size to assure adequate power to this clinical trial was calculated considering differences of at least 1 mm for CAL and a standard deviation of 1.1 mm between groups in initially deep periodontal pockets (> 6 mm).6 Based on these calculations, it was determined that =19 subjects per group would be necessary to provide an 80% power with an a of 0.05.
RESULTS
Subject retention
Ninety-eight subjects were assessed for their eligibility before entering the study. Of these, 22 subjects were excluded because they did not meet the inclusion criteria. Of the 76 subjects recruited, 72 patients had complete data for all three monitoring visits, while two subjects had one missing visit. Intent-to-treat analyses were performed in the 2 subjects with missing data, whereby the last observation was carried forward, providing a total of 74 subjects with complete data that were included in the analyses. The remaining two subjects (one from each group) had baseline data only and their data were not included in the analyses. Participation of individuals during the study is illustrated in figure 1.
Adverse events
All the subjects who completed the study reported full adherence to the prescribed course of antibiotics. Seven subjects from the CIPRO + MET group reported adverse events during the study, such as diarrhea and nausea. Moreover, three subjects from the CIPRO + MET group reported dizziness. Most subjects from the MOX group affirmed that the medications did not cause any major disturbance in their daily routine.
Clinical findings
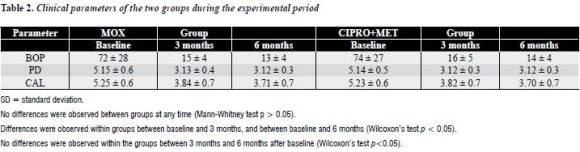
Table 1 presents the baseline clinical and demographic characteristics of the 76 patients subdivided into the two treatment groups. There were no statistically significant differences among treatment groups for any of the parameters except for age.
Table 2 displays descriptive statistics and comparisons for both groups concerning BOP, PD and CAL. No differences were observed between the groups at any timepoint. When comparisons were made within each group, both groups resulted in significant reduction of PD and BOP compared with baseline (P < 0.001), and this difference was maintained at 6 months from baseline in both groups. No differences were observed within the groups between 3 months and 6 months after baseline. When comparisons were made within each group, both groups exhibited significant improvement in CAL compared with baseline (P < 0.001), and this difference was maintained at 6 months from baseline in both groups. In the same way, no differences were observed within the groups between 3 months and 6 months after baseline.
Additional differences concerning the effect of different treatments on the primary outcome were sought with the site instead of the subject as the observational unit, analyzing the subset of pockets with PD > 6 mm (table 3). According to the findings, subjects in both groups at 6 months displayed the greatest reduction from baseline in proportions of sites with PD > 6 mm (P < 0.001). No differences were observed between the subjects who received adjunctive MOX or CIPRO + MET.
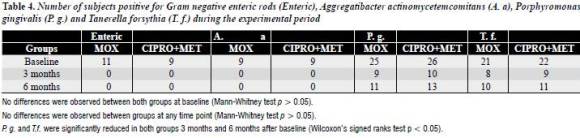
Microbiological findings
The number of subjects positive for investigated species is presented in table 4. At baseline no differences were observed between both groups. Likewise, no differences were observed between groups at any timepoint. Administration of antimicrobials resulted in elimination and reduction of most bacteria, at the subject level, 3 months after baseline. GNER and A. actinomycetemcomitans were not identified in both groups 3 months after baseline, and P. gingivalis and T. forsythia were significantly reduced in both groups 3 months after baseline (P < 0.05). Six months from baseline, a recolonization of investigated species was observed, except for GNER and A. actinomycetemcomitans. However, P. gingivalis and T. forsythia were reduced significantly from baseline (P < 0.05).
DISCUSSION
This randomized, clinical trial evaluated the clinical and microbiological effects of SRP in combination with adjunctive antibiotics (MOX versus CIPRO+MET) in the treatment of subjects with chronic periodontitis associated with GNER. These bacteria are often recovered from the subgingival microbiota of patients considered being clinically refractory to mechanical and conventional antibiotic periodontal therapies.12-15 The high pathogenic potential of GNER10 together with the high proportions in refractory lesions10, 12-15 implicate them in the pathogenesis of various forms of periodontitis.17
This suggests the need for appropriate antibiotherapy in treating periodontal infections involving GNER.10, 12-15 Moreover, GNER can be key pathogens in severe infections of the lower respiratory tract, skin, bone and joints, urinary tract, and various body sites8, 11, 28 and they elaborate a number of potent enzymes and toxins of possible importance for destruction of periodontal tissues.11
The adjunctive use of MOX or ciprofloxacin was selected based on earlier in vitro7, 17-21 and in vivo4, 10 studies that suggested the clinical and microbiological benefits of these antibiotics in the treatment of periodontitis associated with GNER. To the best of our knowledge, the present study is the first clinical study comparing MOX and CIPRO + MET as an adjunct to SRP.
Clinical data
Both therapies used in the present study improved the clinical parameters evaluated. SRP combined with either MOX or with CIPRO + MET was equally effective in improving PD and CAL. The same pattern was observed for the BOP parameter and no differences were observed between groups at any time on the subject level. To our knowledge, only two studies to date have independently investigated the efficacy of adjunctive moxifloxacin4 and ciprofloxacin alone10 in periodontitis. With this in mind, our results are in agreement with Guentsch et al4 who showed an enhanced clinical response when MOX was combined with SRP on the treatment of chronic periodontitis. On the other hand, it is important to note that patients included in the study with ciprofloxacin10 had a history of refractory periodontitis associated with GNER. This finding is especially interesting because thorough mechanical treatment was unable to completely suppress these organisms in infected periodontitis lesions.10 Similar to the current investigation, adjunctive systemic ciprofloxacin therapy demonstrated improvements in BOP, PD and CAL. The results of the current investigation are in accord with studies in the literature that suggest that systemic metronidazole alone or combined significantly improved clinical outcomes of non-surgical periodontal debridement.1-3,6 Interestingly, Cionca et al1 recently showed that the addition of antimicrobial agents to thorough mechanical treatment may reduce the need for further treatment.
When analyzing data at a site level, and in particular, the proportion of sites with PD >6 mm, a similar pattern was observed between groups. MOX group and CIPRO + MET group exhibited a significant reduction of the percentages of sites > 6 mm. This finding indicates a beneficial effect of these antimicrobials on deep pockets. Conclusions of the present study agree with a recently published randomized clinical trial by Guentsch et al.4 These authors have shown that a 7-day adjunctive course of systemic MOX resulted in an additional reduction of PD in deep pockets (> 6 mm) at 6 months also in chronic periodontitis. Similar results at 12 months were shown for ciprofloxacin in patients with refractory periodontitis harboring GNER.10 Both systematic reviews evaluating the effects of systemically administered antibiotics suggested that antibiotics provided greater benefit in subjects with more periodontal disease and at deeper periodontal sites.2, 3
Microbiological data
This is the first clinical study to date that has evaluated the microbiological changes that occur with the use of CIPRO+MET and the second one that has evaluated MOX in the treatment of periodontal disease, and the first one that has directly compared these two treatment protocols. We focused on determination of GNER rods and three periodontopathogens (A. actinomycetemcomitans, P. gingivalis and T. forsythia). The reasons for studying GNER were explained previously. On the other hand, it has been shown that a decrease in the counts of periodontopathogens studied in this investigation is associated with clinical improvements and these microorganisms are predictors for therapy results.5
Our previous paper20 reported four species of GNER in subgingival plaque in 20 (26.31%) of 76 patients: K. pneumoniae occurred in 12 patients, Pseudomonas aeruginosa in four patients and three other species were recovered with lower prevalence. In Latin America their occurrence varies amply within studied populations, reaching a prevalence of 17.6% in Chile29 and 67% in Santo Domingo.30 Similar frequencies to those encountered in our study20 were reported among Brazilians18 and Colombians.19, 29
Likewise, the occurrence of periodontopathogens observed in this study was similar to previous investigations.19, 29
It is important to note that colonies of GNER are bigger in size19 indicating that these organisms could colonize the periodontal pockets in high proportions. On the other hand, polymerase chain reaction (PCR) detection does not take into consideration whether the sample is viable, and thus may yield a higher frequency.19 D’Ercole et al31 recently compared conventional culture methods and multiplex PCR for the detection of periodontopathogenic bacteria and observed that for both methods, there was a good degree of accuracy in the determination of A. actinomycetemcomitans and P. gingivalis. Similar results were obtained for T. forsythia.32 Both culture and PCR techniques introduced many methodological problems when applied in oral microbiology, but the ideal technique for accurate detection of pathogens in subgingival plaque samples has yet to be developed.31 As with Botero et al,19 the present study reports on the occurrence of the microorganisms detected based on culture techniques because it allows us later to work with the cultured microorganisms. Further studies are required in order to clarify the effect of GNER on clinical parameters and response to periodontal treatment.
In agreement with the clinical results, the two groups presented favorable changes in the subgingival microbial profile after treatment. This observation was consistent with several studies,1, 4, 6 where a diminution in occurrence of the test species was significantly associated with an improvement in CAL and a decrease in PD. The most marked decrease in prevalence occurred between the baseline visit and the three months sampling. These results are in agreement with other studies that also demonstrated the adjunctive effects of these antibiotics, in reducing red complex species1,4-6 and GNER.10 Interestingly, in the current study, both adjunctive antibiotic protocols reduced subgingival GNER and A. actinomycetemcomitans to undetectable levels, after three and six months. This observation is consistent with the findings described by Slots et al10 and Guentsch et al,4 who found out that GNER and A. actinomycetemcomitans were reduced to undetectable levels after adjunctive ciprofloxacin and MOX respectively. As it was observed in previous studies,1, 4-6 for P. gingivalis and T. forsythia a slight rebound was detected after three months.
The treated subjects in the current study exhibited a good clinical response, suggesting that a rapid decrease in subgingival counts of periodontopathogens and GNER may be crucial for successful periodontal therapy and long-term periodontal stability.5, 10 The longitudinal follow-up of these subjects will help to clarify this hypothesis.
The present GNER and periodontopathogens investigated were highly susceptible to the fluoroquinolones ciprofloxacin and moxifloxacin.20, 22 Observed concentrations of ciprofloxacin in gingival crevicular fluid of about 2.5 Μg/mL are many times mores elevated than levels normally obtained in plasma.33 This may be due to improvement of quinolones in polymorphonuclear granulocytes.33 In therapeutic doses, the drug appears in saliva at or lower than levels achieved in serum.33 Its low activity against Gram-negative anaerobes usually limits its use in periodontics to combinations with metronidazole.10 In contrast, the novel 8-methoxy quinolone moxifloxacin has effective antimicrobial action against both Gram-negative and Grampositive anaerobes.20, 22 Müller et al34 showed that all tested strains of A. actinomycetemcomitans were susceptible to moxifloxacin at 0.032 Μg/mL. Concentrations in saliva and capillary plasma narrowly reflect equivalent concentrations in venous plasma, but primarily surpass plasma levels.33 Quinolones are recognized for their modulation of the immune reaction, permitting the in vitro extermination of A. actinomycetemcomitans by polymorphonuclear leukocytes.35
Adverse events
Ten subjects (26.3%) from the CIPRO+MET group reported adverse events during the study. These results are in agreement with a systematic review2 evaluating the effects of systemically administered antibiotics which suggested that 39% of subjects in the test group exhibited diarrhea when provided with metronidazole alone or combined. Moreover, ciprofloxacin has been associated with rare but clinically important adverse events in patients with impaired renal function36 and various cases of “Torsades de Pointes” have been reported in the United States.37 Conversely, subjects from the MOX group did not report adverse events during this investigation, corroborating the results of Guentsch et al.4
In addition to antimicrobial activity studies, the properties of moxifloxacin have been studied, showing excellent bioavailability, long half-life and good tissue penetration of this drug.38 Furthermore, it has an excellent tolerability.4 The pharmacokinetic properties allow a single dose treatment per day. This reduces costs and enhances the patient’s compliance.39 This is an important fact, because incomplete adherence to a 7-day adjunctive course of systemic antibiotics is associated with decreased clinical outcomes in subjects with periodontitis.40
The current study did have limitations. It was a randomized study, but it was not placebo controlled. Supplying placebo tablets was considered, but with the diverse dosing regimes and the dissimilar variety of tablets/capsules implicated, composing a standard placebo offered some complexity. It was therefore decided not to use placebos. However, the examining clinician was unaware of the treatment group to which subjects were assigned. Similar situations were presented by Haffajee et al6 and Guentsch et al.4 in two recent clinical trials. Another group including only mechanical treatment was not included in the present study. The authors believe it is clear that the administration of systemic antibiotics during mechanical periodontal treatment is widely reported and accepted by distinguished researches during the treatment of patients with chronic periodontitis harboring GNER.10,12-15 Moreover, two systematic reviews showed that adjunctive use of systemically administered antibiotics provided a better clinical outcome, particularly in terms of PD reduction and attachment-level gain, than SRP, not only in aggressive forms of periodontitis but also in subjects with chronic periodontitis.2,3 Finally, it was recently reported that the addition of antimicrobial agents to thorough mechanical treatment may reduce the need for further treatment.1 Therefore, a mechanical-treatment-only group was not used in the present protocol. Despite its limitations, a prospective decision to incorporate adjunctive MOX or CIPRO-MET as a treatment option is confirmed by the outcomes of the present study.
The current protocols for antibiotic treatment in periodontal therapy are based on microbiological studies conducted outside Latin American countries.
Consequently, the prevalence of considerable differences in the composition of the subgingival flora in diverse geographical populations may increase the possibility of developing antibiotic resistance or promoting a subgingival overgrowth of superinfecting organisms.41 Moxifloxacin offers several advantages over the previously available fluoroquinolones including reduced propensity to promote the development of resistance.11 Although the published data and clinical experience with these agents is limited, given their relatively recent entry into our market, this perspective attempts to provide an understanding of the potential role of moxifloxacin in the treatment of patients with chronic periodontitis harboring enteric rods in subgingival plaque.
CONCLUSIONES
Within the limitations of this study, the results indicate that patients with chronic periodontitis harboring subgingival Gram-negative enteric rods may benefit from systemic administration of moxifloxacin or ciprofloxacin plus metronidazole as an adjunctive antimicrobial therapy to periodontal mechanical treatment. Both treatment strategies resulted in significant gain of clinical attachment level and decrease in periodontal probing depth. The same pattern was observed for BOP. Suppression of GNER below detectable levels for at least 6 months was also demonstrated. Consequently, systemic use of moxifloxacin or ciprofloxacin plus metronidazole as an adjunct to conventional therapy may be an alternative for the treatment of patients with chronic periodontitis. The treatment method should be based on compliance and potential adverse effects.
1. Cionca N, Giannopoulou C, Ugolotti G, Mombelli A. Amoxicillin and metronidazole as an adjunct to full mouth scaling and root planing of chronic periodontitis. J Periodontol 2009; 80: 364-371. [ Links ]
2. Haffajee AD, Socransky SS, Gunsolley JC. Systemic antiinfective periodontal therapy. A systematic review. Ann Periodontol 2003; 8: 115-181. [ Links ]
3. Herrera D, Sanz M, Jepsen S, Needleman I, Roldán S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol 2002; 29 Supl 3: 136-159. [ Links ]
4. Guentsch A, Jentsch H, Pfister W, Hoffmann T, Eick S. Moxifloxacin as an adjunctive antibiotic in the treatment of severe chronic periodontitis. J Periodontol 2008; 79: 1894-1903. [ Links ]
5. Haffajee AD, Patel M, Socransky SS. Microbiological changes associated with four different periodontal therapies for the treatment of chronic periodontitis. Oral Microbiol Immunol 2008: 23: 148-157. [ Links ]
6. Haffajee AD, Torresyap G, Socransky SS. Clinical changes following four different periodontal therapies for the treatment of chronic periodontitis: 1 year results. J Clin Periodontol 2007; 34: 243-253. [ Links ]
7. Müller HP, Heinecke A, Borneff M, Kiencke C, Knopf A, Pohl S. Eradication of Actinobacillus actinomycetemcomitans from the oral cavity in adult periodontitis. J Periodontal Res 1998; 33: 49-58. [ Links ]
8. Gupta A. Hospital-acquired infections in the neonatal intensive care unit-Klebsiella pneumoniae. Semin Perinatol 2002; 26: 340-345. [ Links ]
9. Slots J, Feik D, Rams TE. Age and sex relationships of superinfecting microorganisms in periodontitis patients. Oral Microbiol Immunol 1990; 5: 305-308. [ Links ]
10. Slots J, Feik D, Rams TE. Prevalence and antimicrobial suceptibility of Enterobacteriaceae, Pseudomonadaceae and Acinetobacter in human periodontitis. Oral Microbiol Immunol 1990; 5: 49-154. [ Links ]
11. Gonçalves MO, Coutinho-Filho WP, Pimenta FP et al. Periodontal disease as reservoir for multi-resistant and hydrolytic enterobacterial species. Lett Appl Microbiol 2007; 44: 488-494. [ Links ]
12. Listgarten MA, Lai CH, Young V. Microbial composition and pattern of antibiotic resistance in subgingival microbial samples from patients with refractory periodontitis. J Periodontol 1993; 64: 155-161. [ Links ]
13. Listgarten MA, Lai CH. Comparative microbiological characteristics of failing implants and periodontally diseased teeth. J Periodontol 1999; 70: 431-437. [ Links ]
14. Edwardsson S, Bing M, Axtelius B, Lindberg B, Söderfeldt B, Attström R. The microbiota of periodontal pockets with different depths in therapy-resistant periodontitis. J Clin Periodontol 1999; 26: 143-152. [ Links ]
15. Slots J, Rams TE, Listgarten MA. Yeasts, enteric rods and pseudomonads in the subgingival flora of severe adults periodontitis. Oral Microbiol Immunol 1988; 3: 47-52. [ Links ]
16. Slots J, Rams TE, Schonfeld SE. In vitro activity of chlorhexidine against enteric rods, pseudomonads and acinetobacter from human periodontitis. Oral Microbiol Immunol 1991; 6: 62-64. [ Links ]
17. of enteric rods and pseudomonads from advanced adult periodontitis. Oral Microbiol Immunol 1990; 5: 298-301. [ Links ]
18. Barbosa FC, Mayer MP, Saba-Chujfi E, Cai S. Subgingival occurrence and antimicrobial susceptibility of enteric rods and pseudomonads from Brazilian periodontitis patients. Oral Microbiol Immunol 2001; 16: 306-310. [ Links ]
19. Botero JE, Contreras A, Lafaurie G, Jaramillo A, Betancourt M, Arce RM. Occurrence of periodontopathic and superinfecting bacteria in chronic and aggressive periodontitis subjects in a Colombian population. J Periodontol 2007; 78: 696-704. [ Links ]
20. Ardila CM, Fernández N, Guzmán IC. Antimicrobial susceptibility of moxifloxacin against gram-negative enteric rods from Colombian patients with chronic periodontitis. J Periodontol 2010; 81: 292-299. [ Links ]
21. Watt B, Brown FV. The in vitro activity of ciprofloxacin in combination with other agents against anaerobes of clinical interest. J Antimicrob Chemother 1986; 17: 679-680. [ Links ]
22. Ardila CM, Granada MI, Guzmán IC. Antibiotic resitance of subgingival species in chronic periodontitis patients. J Periodontal Res 2010; 45: 557-563. [ Links ]
23. Araujo MW, Hovey KM, Benedek JR, et al. Reproducibility of probing depth measurement using a constant-force electronic probe: analysis of inter- and intraexaminer variability. J Periodontol 2003;74: 1736-1740. [ Links ]
24. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999; 4: 1-6. [ Links ]
25. Möller AJ. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr 1966; 74 Supl: 1-380. [ Links ]
26. Slots J. Rapid identification of important periodontal microorganisms by cultivation. Oral Microbiol Immunol 1986; 1: 48-57. [ Links ]
27. Slots J, Reynolds HS. Long-wave UV light fluorescence for identification of black-pigmented Bacteroides spp. J Clin Microbiol 1982; 16: 1148-1151. [ Links ]
28. Al-Anazi KA, Al-Jasser AM, Al-Zahrani HA, Chaudhri N, Al-Mohareb FI. Klebsiella oxytoca bacteremia causing septic shock in recipients of hematopoietic stem cell transplant: Two case reports. Cases J 2008; 18: 160-163. [ Links ]
29. Herrera D, Contreras A, Gamonal J, Oteo A, Jaramillo A, Silva N, et al. Subgingival microbial profiles in chronic periodontitis patients from Chile, Colombia and Spain. J Clin Periodontol 2008; 35: 106-113. [ Links ]
30. Slots J, Rams TE, Feik D, Taveras HD, Gillespie GM. Subgingival microflora of advanced periodontitis in the Dominican Republic. J Periodontol 1991; 62: 543-547. [ Links ]
31. DErcole S, Catamo G, Tripodi D, Piccolomini R. Comparison of culture methods and multiplex PCR for the detection of periodontophatogenic bacteria in biofilm associated with severe forms of periodontitis. New Microbiol 2008; 31: 383-391. [ Links ]
32. Boutaga K, van Winkelhoff AJ, Vandenbroucke-Grauls CM, Savelkoul PH. Periodontal pathogens: a quantitative comparison of anaerobic culture and real-time PCR. FEMS Immunol Med Microbiol 2005; 45: 191-199. [ Links ]
33. Conway TB, Beck FM, Walters JD. Gingival fluid ciprofloxacin levels at healthy and inflamed human periodontal sites. J Periodontol 2000; 71: 1448-1152. [ Links ]
34. Müller HP, Holderrieth S, Burkhardt U, Höffler U. In vitro antimicrobial susceptibility of oral strains of Actinobacillus actinomycetemcomitans to seven antibiotics. J Clin Periodontol 2002; 29: 736-742. [ Links ]
35. Dalhoff A. Immunomodulatory activities of fluoroquinolones. Infection 2005; 33(Suppl. 2): 55-70. [ Links ]
36. Iannini PB. The safety profile of moxifloxacin and other fluoroquinolones in special patient populations. Curr Med Res Opin 2007; 23: 1403-1413. [ Links ]
37. Frothingham R. Rates of torsades de pointes associated with ciprofloxacin, ofloxacin, levofloxacin, gatifloxacin, and moxifloxacin. Pharmacotherapy 2001; 21: 1468-1472. [ Links ]
38. Cachovan G, Nergiz I, Thuss U et al. Penetration of moxifloxacin into rat mandibular bone and soft tissue. Acta Odontol Scand 2009; 67: 182-186. [ Links ]
39. Krasemann C, Meyer J, Tillotson G. Evaluation of the clinical microbiology profile of moxifloxacin. Clin Infect Dis 2001; 32 Supl 1: S51-63. [ Links ]
40. Guerrero A, Echeverría JJ, Tonetti MS. Incomplete adherence to an adjunctive systemic antibiotic regimen decreases clinical outcomes in generalized aggressive periodontitis patients: a pilot retrospective study. J Clin Periodontol 2007; 34: 897-902. [ Links ]
41. Pinheiro ET, Gomes BP, Drucker DB, Zaia AA, Ferraz CC, Souza-Filho FJ. Antimicrobial susceptibility of Enterococcus faecalis isolated from canals of root filled teeth with periapical lesions. Int Endod J 2004; 37: 756-763. [ Links ]











 text in
text in