Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Odontología Universidad de Antioquia
Print version ISSN 0121-246X
Rev Fac Odontol Univ Antioq vol.23 no.2 Medellín Jan./June 2012
ORIGINAL ARTICLES DERIVED FROM RESEARCH
Adjunctive systemic administration of moxifloxacin versus ciprofloxacin plus metronidazole in the treatment of chronic periodontitis harboring Gram-negative enteric rods: II. A multilevel analysis1
Isabel Cristina Guzmán2; Hugo Grisales Romero3; Carlos Martín Ardila Medina4
1 This study was sponsored by a grant from the National School of
Public Health and the Epidemiology Group of Universidad de Antioquia,
Colombia. The authors report no conflicts of interest related to this
study
2 Periodontist. Assistant Professor, School of Dentistry, Universidad de
Antioquia, Colombia
3 PhD in Epidemiology. Associate Professor, National School of Public
Health, Universidad de Antioquia, Colombia
4 Periodontist, PhD, Associate Professor, School of Dentistry, Universidad
de Antioquia, Colombia. Head of the Biomedical Stomatology Group,
Universidad de Antioquia, Colombia
SUBMITTED: SEPTEMBER 13/2011 - ACCEPTED: OCTOBER 13/2011
Guzmán IC, Grisales H, Ardila CM. Adjunctive systemic administration of moxifloxacin versus ciprofloxacin plus metronidazole in the treatment of chronic periodontitis harboring Gram-negative enteric rods: II. A multilevel analysis. Rev Fac Odontol Univ Antioq 2012; 23(2): 207-224.
ABSTRACT
INTRODUCTION: the site, tooth, and patient levels are involved in the periodontal inflammatory process. The purpose of this study was to compare the effect of site, tooth, and patient-related factors on the success of scaling and root planing combined with systemic administration of moxifloxacin or ciprofloxacin plus metronidazole in the treatment of subjects with chronic periodontitis harboring Gramnegative enteric rods.
MATERIALS AND METHODS: seventy-six patients participated in this randomized clinical trial, divided into two groups. The subjects were
treated with scaling and root planing plus adju nctive moxifloxacin (moxifloxacin group; n = 38) or scaling and root planing plus adjunctive
ciprofloxacin plus metronidazole (ciprofloxacin plus metronidazole group; n = 38). Periodontal and microbiological data were recorded at baseline and at 3 and 6 months after treatment. The relative contribution of patient, tooth and site-associated parameters was evaluated
with a hierarchical multilevel model.
RESULTS: most of the variance was attributed to site level (73%), followed by tooth level (18.1%) and
patient level (8.9%). The multilevel analysis associated probing depth reductions with subject factors (smoking status and treatment), tooth
factors (tooth type), and site factors (mesial-distal location). Probing depth reduction was significantly smaller in smokers. Both treatment
protocols significantly reduced probing depth. Anterior teeth responded better than posterior teeth. At the site level, greater reductions were
observed at interdental sites. The presence of plaque and bleeding on probing at the tooth site level had a significant negative impact on the
outcome of chronic periodontitis harboring Gram-negative enteric rods.
CONCLUSIONS: smoking habits, tooth type, mesial-distal location,
plaque and bleeding on probing at site level were significant factors in determining the clinical outcome of scaling and root planing plus
adjunctive antibiotic treatment in chronic periodontitis harboring Gram-negative enteric rods.
Key words: antimicrobial(s), periodontitis, multilevel analysis.
INTRODUCTION
It is generally accepted that scaling and root planing (SRP) is an effective periodontitis treatment approach; however, it is also evident that various subject-related and tooth site-related factors may compromise the healing response to treatment.1-5
Recent systematic reviews have shown that adjunctive use of systemically administered antibiotics provided a better clinical outcome, particularly in terms of probing depth (PD) reduction and attachment-level gain than SRP in subjects with chronic periodontitis.6, 7 However, these systematic reviews, like many other previous studies on this issue,8-12 use mean values and are not able to reflect the benefit of a particular technique on teeth with certain characteristics.
Gram-negative enteric rods (GNER) are opportunistic pathogens in a wide range of human infections13 and they can be detected in the subgingival environment of periodontitis subjects.14-25 These microorganisms are able to produce virulence factors and have shown the capacity to invade human tissue.16 Subgingival GNER often persist after periodontal debridement and surgery and have been implicated as key pathogens in cases of refractory periodontitis.15, 17-20 These microorganisms were detected at higher frequency and in higher proportions in patients with failing implants.18 Additionally, they show less susceptibility to chlorhexidine,21 and the fact that these microorganisms exhibit in vitro resistance to the majority of adjunctive antibiotics used to treat periodontitis22-25 means that periodontal lesions associated with these organisms do not respond to conventional treatment modalities.15 Ciprofloxacin therapy has the potential to eradicate GNER from periodontal pockets but it has little activity against the main periodontal pathogens;15, 26 therefore, it should be combined with an antimicrobial agent active against anaerobes, such as metronidazole.14, 15 Conversely, in previous articles, moxifloxacin (MOX) has shown in vitro activity against GNER,25 and in vitro27 and in vivo8 efficacy against periodontopathogens. These studies have also outlined a procedure for clinical trials to investigate the effects of MOX in the treatment of patients with chronic periodontitis harboring GNER in subgingival plaque.
Application of multilevel analysis, which takes the clustering effect of periodontal research data into consideration, may supply a more precise elucidation of the accepted hierarchical structure of the clinical findings of periodontitis and the healing responses subsequent to periodontal treatment.1-5 Lately, several investigations have adopted such an approach in their periodontal trial data analysis.1-5 However, it is also notable that to date no research has focused on the topic of relative contribution of patient, tooth, and site-specific parameters in establishing the reported variability of outcomes of SRP combined with systemic antibiotics.
Therefore, the purpose of this study was to evaluate, by means of a multilevel analysis, the relative importance of patient, tooth, and site-related factors in determining the clinical outcomes of SRP combined with systemic MOX or ciprofloxacin plus metronidazole (CIPRO + MET) in the treatment of subjects with chronic periodontitis harboring Gram-negative enteric rods.
MATERIALS AND METHODS
Subjects
Seventy-six systemically healthy subjects (45 women and 31 men), aged 27 to 66 years who attended the dental clinics of Universidad de Antioquia were recruited from October 2008 to March 2009. Informed and written consent was obtained from each participant.
The study design was approved by the Ethics Committee on Human Research of Universidad de Antioquia's University Investigation Department, according to the Declaration of Helsinki on experimentation involving human subjects. Patients with a diagnosis of chronic periodontitis were considered candidates for the study. Subjects were > 26 years of age, had at least 20 natural teeth, including at least 1 molar tooth in each quadrant, and at least eight sites with PD ≥ 5 mm. Exclusion criteria included allergy to antibiotics, diabetes, cardiovascular disease, or any other systemic disease that could alter the course of periodontal disease. Pregnant or nursing women, consumption of systemic antimicrobials or anti-inflammatory drugs in the last six months, and periodontal therapy during the last six months also served as exclusion criteria.
Experimental design and treatment
This was a randomized trial with masking of examiner, clinician performing treatment and statistician, with 6 months of follow-up. Subjects were randomly assigned by a computer-generated table to receive one of the two treatments. The assignment of subjects to the treatment groups was carried out by the clinic coordinator remote from the study. The randomization code was held centrally by the clinic coordinator and was not broken until completion of the data analysis. The two treatment groups consisted of SRP combined with systemically administered MOX at the dosage of 400 mg once daily for 7 days (MOX group) or SRP combined with systemically administered ciprofloxacin (at the dosage of 1g once daily) plus metronidazole (500 mg b.i.d. for 7 days) (Ciprofloxacin + Metronidazole group). One-stage full-mouth SRP under local anesthesia was performed in approximately two and half hours by the same experienced periodontist. The endpoint of SRP was a tactile smooth root surface. The adjunctive agents were started at the SRP visit. Subjects in the MOX and CIPRO + MET groups were extensively informed about the intake of the prescribed medication.
Subjects were clinically and microbiologically monitored at baseline (before therapy) and at 3 and 6 months post-therapy.
During the monitored sessions, oral hygiene was evaluated and home care instructions were reemphasized. All subjects came for recall visits and received oral hygiene evaluations. The recall visits were made on a 2-week interval during the 6 months after treatment.
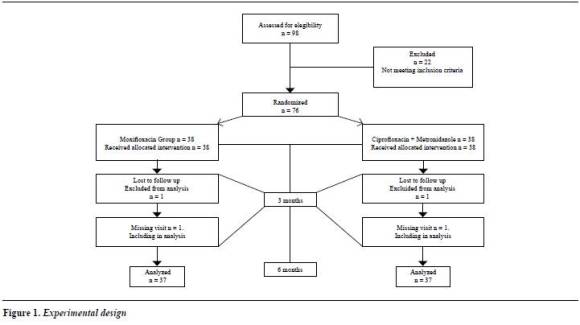
The experimental design is presented in figure 1.
Compliance
A dental assistant telephoned each subject during the next 6 days to remind them about taking the remaining doses. The same dental assistant, not involved in the randomization process, recorded compliance with medication intake and occurrence of adverse events. The subjects were asked to bring the boxes containing the medication the week after the SRP visit, when the pills were counted in order to check any inaccuracy in drug taking.
Clinical evaluation
Site parameters: by using a calibrated standard probe, PD was measured at six sites per tooth (mesiobuccal, buccal, distobuccal, distolingual, lingual and mesiolingual) in all the teeth except third molars. PD was obtained to the closest millimeter, by means of a calibrated standard probe (UNC-15, Hu-Friedy, Chicago, IL). In all the visits, the measurements (PD, CAL, BOP, plaque) were taken by the same blinded, trained, calibrated clinician (this examiner was different to the clinician performing treatments) at baseline and 3 and 6 months after periodontal treatment. Presence of plaque and bleeding on probing (BOP) were also assessed at each site. The clinician making the clinical measurements did not perform therapy on the subjects. Intra-examiner reproducibility was assessed before and during the experimental period, according to the method described by Araujo et al.28 Repeated measurements were performed on 10 periodontal patients (who were not participating in this study), five of whom were examined immediately before the clinical trial, and the other five during the experimental period. Duplicate measurements were conducted in each patient with at least 2 h between one examination and the other. The intra-class correlation coefficients for mean PD and CAL were 0.92 and 0.91, respectively. The examiners' reproducibility of measurements was similar before and during the study. Diagnosis of chronic periodontitis was made based on the criteria defined by the American Academy of Periodontology (AAP).29
Tooth parameters: furcation involvement was graded using a Nabers probe. A tooth type categorical variable was also included in the analysis (molars, premolars and incisors).
Subject parameters: age, sex, smoking status, treatment, and presence of GNER, Aggregatibacter actinomycetemcomitans (Aa), Porphyromonas gingivalis (Pg) and Tanerella forsythia (Tf) were included in the multilevel analysis.
Microbial sampling
Microbial sampling on periodontitis patients was performed on pockets ≥ 5 mm. The deepest six pockets of each patient were selected for sampling. After removing supragingival plaque (after periodontal examination) with curettes and isolating the area with cotton rolls, sterile paper points (Maillefer, Ballaigues, Switzerland) were inserted into each periodontal pocket for 20 seconds. The paper points were transferred to a tube with Viability Medium Göteborg Anaerobically (VGMA) III medium.30 The samples were analyzed by using microbial culture techniques for the presence of periodontopathic bacteria according to Slots.31 All the samples were processed in ≤ 24 hours at room temperature and immediately incubated in CO2 and anaerobic culture systems. Brucella blood agar medium was incubated at 35°C in an anaerobic jar for 7 days. Trypticase-soy with serum, bacitracin, and vancomycin medium was incubated in 10% CO2 in air at 37°C for 4 days. Presumptive identification was performed according to the methods described31, 32 and using commercial identification micromethod systems (RapID ANA, Remel, Norcross, GA) for A. actinomycetemcomitans, P. gingivalis and T. forsythia. Total viable count (TVC) was defined as the total number of colony-forming units obtained on non-selective media plates. Species found on selective media were enumerated and their percentage of TVC was calculated.
Isolation of Gram-negative enteric rods by culture. After placement for 20 seconds, the paper points were pooled into a vial containing 2.0 ml of VMGA III transport medium.30 The sample vials were maintained at room temperature, transferred to the laboratory and processed within 4 h after sampling.
After the vials were placed in an incubator for 30 min at 37 °C, bacterial plaque was mechanically dispersed with a test tube mixer at the maximal setting for 60 s. Serial 10-fold dilutions were prepared in pepton water, and aliquots were plated on Mac- Conkey agar. The plates were incubated aerobically at 37 °C for 24 h. Each isolate was characterized according to colonial and cellular morphology and Gram-stain characteristics. GNER were classified using a standardized biochemical test (BD, Sparks, MD). TVC was defined as the total number of colony-forming units obtained on non-selective media plates. Species found on selective media were enumerated and their percentage of TVC was calculated.
Each patient provided a pooled subgingival plaque sample. Equal numbers of isolates were used from each subject.
Statistical analysis
Data were entered into an Excel (Microsoft Office 2007) database and were proofed for entry errors. Normal distribution of continuous variables was verified with the Kolmogorov-Smirnov test with Liliefors correction. Categorical data were analysed with the X 2 test, and the percentage data between the two groups were compared with the Mann-Whitney test. Differences between groups and between different timepoints within groups were tested by the Mann-Whitney test and the Wilcoxon signed rank test, respectively. A significance level of 0.0005 was set for all the tests.
The influence of different factors on the outcome was examined with multilevel regression analyses. A three-level random intercept regression model was constructed: tooth site at level 1, tooth at level 2 and subject at level 3.This technique allows identification of single-tooth effects or parameters while still considering the individual patient as a statistical unit and the dependencies of site data within a patient.
A variance components model (null model) was constructed using changes in PD between baseline and six months as the dependent variable (ΔPD) but without inserting explanatory variables.
The null model was used to estimate the overall variability of ΔPD and to attribute it to the patient, tooth and site levels. The normality assumption of ΔPD was verified and multi-collinearity was performed. Subsequently, a series of explanatory variables were entered into the model (covariate model). This further step allowed examination of the relationship between each covariate and the dependent variable. The fit change of each model (-2log likelihood) including /excluding explanatory variables was calculated and the significance was tested by chi-square analysis.
All data handling and statistical testing were performed with the same software package (SPSS, Statistical Package for the Social Sciences, version 15, Chicago, IL). A statistical package specifically designed for multilevel modeling (Multilevel Models Project Institute of Education, MLwiN, version 2.16, London, U.K) was used to determine the influence of subject, tooth and site-related covariates on the outcome variables.
Sample size calculation
Initially, sample size calculations were based on subjects, as the analysis unit for randomized studies. The ideal sample size to assure adequate power to this clinical trial was calculated considering differences of at least 1 mm for CAL and a standard deviation of 1.1 mm between groups in initially deep periodontal pockets (> 6 mm).12 Based on these calculations, it was determined that at least 19 subjects per group would be necessary to provide an 80% power with an Α of 0.05. With multiple follow-up measures and the intention-to-analyze sites within a multilevel framework, study-size estimates based on subject-level t-tests were anticipated to be overly conservative. Thus, a correction factor was applied that can be used to calculate the required sample size in multilevel studies.33 Based on this correction factor, it can be calculated that 38 patients per group are needed.
RESULTS
Subject retention, patient compliance and adverse events were discussed in a previous paper.34
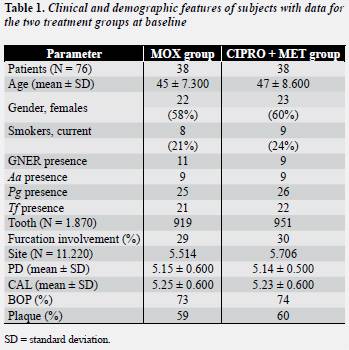
A summary of each level variable is described in table 1 . There were no statistically significant differences between the treatment groups for any of the parameters, except age.
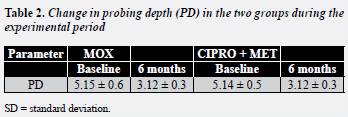
The two therapies used in the present study improved the clinical parameters evaluated. SRP combined with either MOX or with CIPRO + MET was equally effective in improving PD (table 2) .
No differences were observed between the groups (Mann-Whitney test p > 0.05).
Differences within the groups were observed between baseline and 6 months (Wilcoxon's test p < 0.05).
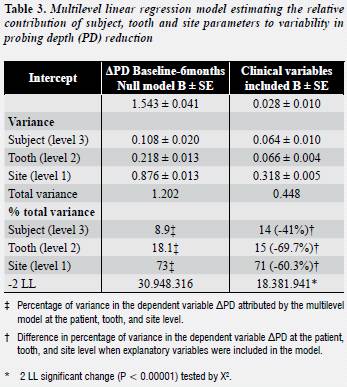
A total of 1,870 teeth and 11,220 sites were included in the multilevel analysis. Results from the variance component multilevel model with ΔPD as the dependent variable, with significant explanatory covariates included, are presented in table 3 . In the null model, variability at each individual level was obtained as a percentage of the total variability calculated, by adding all the estimates together.
The greater variance percentage was attributed to site level (73%), followed by tooth level (18.1%) and patient level (8.9%). On average, adding the tested variables to the model explained 57% of variability at all the levels. Significantly, better fit was obtained by entering all the clinical covariates at all the levels.
Regression estimates and significance testing for all the clinical covariates were also performed. Table 4 shows the regression estimates for covariates. The model confirmed that adjunctive systemic administration of both antibiotic protocols showed a significantly favorable response to periodontal therapy in terms of probing depth changes (P = 0.01). Current smokers (P < 0.00001) showed a significantly less favorable response to periodontal therapy in terms of PD changes. In order to compare PD changes between the groups, a standard multiple testing approach with a Bonferroni correction was used. This analysis yielded a significant difference between smokers and non-smokers in terms of PD changes (0.42 mm ± 0.04, MOX group; 0.40 mm ± 0.04, CIPRO + MET group). No differences were observed between the groups. The only significant variables at the subject level were the presence of GNER and the presence of the periodontopathogens.
At the tooth level, the model demonstrated that anterior teeth showed the most significant PD decreases when compared to posterior teeth (P < 0.00001). Anterior teeth showed significantly greater changes in probing depths after adjunctive MOX or CIPRO + MET than posterior teeth (mean difference 0.14 ± 0.04 mm; P < 0.001 post Hoc comparisons). The CIPRO + MET group showed similar results (mean difference 0.13 ± 0.4 mm; P < 0.001 post Hoc comparisons). No differences were observed between the subjects who received adjunctive MOX compared with subjects who received adjunctive CIPRO + MET. Furcation involvement did not have a significant impact on PD reduction.
At the site level, the model revealed that the mesial and distal sites were the areas with greater PD changes in comparison to the buccal/lingual sites (P = 0.001). The mesial and distal sites showed significantly greater changes in probing depths after adjunctive MOX or CIPRO + MET than buccal or lingual surfaces (mean difference 1.43 ± 0.4 mm; P < 0.001 post Hoc comparisons). The CIPRO + MET group showed similar results (mean difference 1.41 ± 0.4 mm; P < 0.001 post Hoc comparisons). Moreover, at the site level, the presence of plaque (P = 0.002) and BOP (P = 0.004) at baseline were significant in the model.
DISCUSSION
This randomized, clinical trial evaluated the clinical and microbiological effects of SRP in combination with adjunctive antibiotics (MOX versus CIPRO + MET) in the treatment of subjects with chronic periodontitis associated with GNER.
Taking into account the natural hierarchical configuration of data in periodontitis, the present study adopted a multiple regression model to analyze PD reductions by comparing baseline with a 6 month follow- up after SRP combined with systemic MOX or CIPRO + MET in the treatment of subjects with chronic periodontitis. Most of the variance was attributed to site level (73%), followed by tooth level (18.1%) and patient level (8.9%). This implies that most of the variations in periodontal therapy outcomes result from factors acting at the site level. This agrees with previous studies that also assessed the relative contribution of multilevel variation for the outcome of periodontal therapy in clinical trials,1-5 which found out that site-level factors had a much greater impact than subject-level factors. Interestingly, adding the tested variables to the model explained 57% of the variability at all the levels. These results are very similar to those described in a recent publication by Tomasi et al.3 about a clinical trial with multilevel analysis. When explanatory variables were entered into the model, 71% of the variance was attributed to variation between sites. Once again, similar results were reported in clinical trials using multilevel models. 2, 3 The results of the present study associated PD decrease with subject factors (such as smoking habits, or treatment), tooth factors (tooth type) and site factors (mesio-distal position, plaque and BOP), thus confirming the evidence that treatment outcomes at different sites and teeth within the same patient are not independent.1-5 The results of this study also indicate that systemic antibiotics are useful tools in the treatment of smokers with chronic periodontitis, thus confirming previous observations.11, 35 However, non-smoking patients who received adjunctive antibiotics showed the best clinical outcomes, corroborating previous results.11, 35 It is important to note that the smoking status was considered as a dichotomous variable at the subject level.
Future studies could include quantitative measurements, such as number of cigarette packs a year, or analysis of the residual effect of cigarette doses on periodontal health. Another significant patientrelated factor was SRP combined with systemic antibiotics. These results revealed improvement of clinical outcomes with the two approaches, thus supporting other studies that indicate that systemically administered antibiotics provide greater benefit in subjects with more periodontal disease and at deeper periodontal sites.6, 7 Similarly, other authors who used multilevel analysis in evaluations of treatment outcomes demonstrated poorer results of non-surgical therapy.2-4 As pointed out by D'Aiuto et al.,2 it may be expected that by studying patients with significant systemic conditions, more variability may occur.
The data analysis revealed that anterior teeth responded better than posterior teeth. Other multilevel studies have reported similar results after SRP combined with antibiotics.1 Anterior teeth probably heal with more recession and more pocket reduction than posterior teeth.2, 3 In the present study, furcation involvement did not have a significant impact on PD reduction, as shown by two recent clinical trials using multilevel models.1, 5
As occurred in a previous study,2 the divergent outcome is probably due to the fact that in the present study furcation involvement was considered as a tooth-level variable. In contrast to the aforementioned studies, tooth mobility was not included in the current analysis because it yielded divergent results in multilevel modelling.2
At the site level, higher probing depths reductions were observed at the interdental sites, compared to the buccal and lingual sites. These results agree with a previous study11 and are consistent with the frequent finding of deeper pockets in the interproximal areas.2 Furthermore, the presence of plaque and BOP at the tooth site level had a significant negative impact on the outcome. These findings corroborate the observations of recent studies1,5 based on the multilevel analysis of factors associated with the treatment outcome after SRP combined with antibiotics.
They also emphasize the importance of considering factors related to the individual periodontal site when making decisions about the appropriate therapy for those sites with lower response to PD.
The experimental model used in this study required recall visits every two weeks; however, it is important to note that recall appointments are usually assigned every three to six months, and this aspect could have influenced the results.
The first essential step for the treatment of periodontitis is related to the establishment of a diagnosis based on the type of disease, extent, location, and severity. Site level parameters very often include deep pockets, clinical attachment loss, specific periodontal flora, furcation involvement, suppuration, plaque, bleeding on probing, and other factors, such as root ridges, enamel projections, root fractures and deficient restorations that may help clinicians predict the outcome of periodontal therapy. It is also important to note that the progress of periodontal disease may differ from one site to another within the same individual and between individuals. These tooth site-level factors must be used in formulating the prognosis, along with individual-level factors such as smoking, genetic predisposition, age, gender, race, and particular medical conditions. The relative value of site-level factors becomes very important since indicators at this level have demonstrated strong associations with future periodontal attachment loss.36 Similarly, smoking is an important prognostic factor at the subject level, which greatly influences the response to treatment, directly related to a higher proportion of sites that do not respond to periodontal therapy.37 Monitoring these risk factors is critical in the treatment of periodontitis.
Risk information, obtained in a hierarchical manner, bearing in mind the site, tooth and subject levels, facilitates immediate assessment of the periodontal status of an individual and his potential risk of infection and disease progression, and allows therapeutic intervention of the sites under higher risk, as well as the selection of different forms of therapeutic intervention.
This approach is in line with the recommendations by the Consensus Report on epidemiology and diagnosis, 38 which states that screening tests for periodontal disease will need to be targeted to the needs of the patients in a hierarchical manner.
As described in a previous paper, the current study did have limitations, one of them was the information bias due to non-blinding patients; however, by increasing the methodological rigor of other components of the study, the authors attempted to compensate it. Despite these conditions, the decision of incorporating adjunctive MOX or CIPRO + MET as a treatment option is confirmed by the findings of the present study. Additionally, taking into account the cost of quinolones, they appear as an option of inexpensive antibiotics to treat chronic periodontitis harboring Gram-negative enteric rods. Similarly, cost-benefit should be considered.
CONCLUSIONS
Systemic use of moxifloxacin or ciprofloxacin plus metronidazole as an adjunct to antimicrobial therapy may be an alternative for the treatment of patients with chronic periodontitis harboring Gram-negative enteric rods. Both treatment strategies resulted in significant decrease of periodontal probing depth. Multilevel modeling has a great potential as an analytical model for periodontal clinical trials and should be considered in a variety of trial designs due to its contribution to data analysis. On the basis of this investigation, future studies that try to explain our results, in order to verify them, must be performed.
ACKNOWLEDGMENTS
Special thanks to the microbiology laboratory of the school of Dentistry of Universidad de Antioquia for its support.
CORRESPONDING AUTHOR
Carlos M. Ardila
Calle 64 N.° 52-59.
Medellín, Colombia
Phone number 057-4-219 67 60
e-mail: martinardila@gmail.com
REFERENCES
1. Tomasi C, Koutouzis T, Wennström JL. Locally delivered doxycycline as an adjunct to mechanical debridement at retreatment of periodontal pockets. J Periodontol 2008; 79: 431-439. [ Links ]
2. D´Aiuto F, Ready D, Parkar M, Tonetti MS. Relative contribution of patient, tooth, and site-associated variability on the clinical outcomes of subgingival debridement. I. Probing depths. J Periodontol 2005; 76: 398-405. [ Links ]
3. Tomasi C, Leyland AH, Wennström JL. Factors influencing the outcome of non-surgical periodontal treatment: a multilevel approach. J Clin Periodontol 2007; 34: 682-690. [ Links ]
4. Axtelius B, Soéderfeldt B, Attstroém R. A multilevel analysis of factors affecting pocket probing depth in patients responding differently to periodontal treatment. J Clin Periodontol 1999; 26: 67-76. [ Links ]
5. Dannewitz B, Lippert K, Lang NP, Tonetti MS, Eickholz P. Supportive periodontal therapy of furcation sites: non-surgical instrumentation with or without topical doxycycline. J Clin Periodontol 2009; 36: 514-522. [ Links ]
6. Haffajee AD, Socransky SS, Gunsolley JC. Systemic antiinfective periodontal therapy. A systematic review. Ann Periodontol 2003; 8: 115-181. [ Links ]
7. Herrera D, Sanz M, Jepsen S, Needleman I, Roldán S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol 2002; 2 9 Supl 3: 136-159. [ Links ]
8. Guentsch A, Jentsch H, Pfister W, Hoffmann T, Eick S. Moxifloxacin as an adjunctive antibiotic in the treatment of severe chronic periodontitis. J Periodontol 2008; 79: 1894-1903. [ Links ]
9. Cionca N, Giannopoulou C, Ugolotti G, Mombelli A. Amoxicillin and metronidazole as an adjunct to full mouth scaling and root planing of chronic periodontitis. J Periodontol 2009; 80: 364-371. [ Links ]
10. Haffajee AD, Patel M, Socransky SS. Microbiological changes associated with four different periodontal therapies for the treatment of chronic periodontitis. Oral Microbiol Immunol 2008: 23: 148-157. [ Links ]
11. Haffajee AD, Torresyap G, Socransky SS. Clinical changes following four different periodontal therapies for the treatment of chronic periodontitis: 1 year results. J Clin Periodontol 2007; 34: 243-253. [ Links ]
12. Müller HP, Heinecke A, Borneff M, Kiencke C, Knopf A, Pohl S. Eradication of Actinobacillus actinomycetemcomitans from the oral cavity in adult periodontitis. J Periodontal Res 1998; 33: 49-58. [ Links ]
13. Gupta A. Hospital-acquired infections in the neonatal intensive care unit-Klebsiella pneumoniae. Semin Perinatol 2002; 26: 340-345. [ Links ]
14. Slots J, Feik D, Rams TE. Age and sex relationships of superinfecting microorganisms in periodontitis patients. Oral Microbiol Immunol 1990; 5: 305-308. [ Links ]
15. Slots J, Feik D, Rams TE. Prevalence and antimicrobial susceptibility of Enterobacteriaceae, Pseudomonadaceae and Acinetobacter in human periodontitis. Oral Microbiol Immunol 1990; 5: 149-154. [ Links ]
16. Gonçalves MO, Coutinho-Filho WP, Pimenta FP, et al. Periodontal disease as reservoir for multi-resistant and hydrolytic enterobacterial species. Lett Appl Microbiol 2007; 44: 488-494. [ Links ]
17. Listgarten MA, Lai CH, Young V. Microbial composition and pattern of antibiotic resistance in subgingival microbial samples from patients with refractory periodontitis. J Periodontol 1993; 64: 155-161. [ Links ]
18. Listgarten MA, Lai CH. Comparative microbiological characteristics of failing implants and periodontally diseased teeth. J Periodontol 1999; 70: 431-437. [ Links ]
19. Edwardsson S, Bing M, Axtelius B, Lindberg B, Söderfeldt B, Attström R. The microbiota of periodontal pockets with different depths in therapy-resistant periodontitis. J Clin Periodontol 1999; 26: 143-152. [ Links ]
20. Slots J, Rams TE, Listgarten MA. Yeasts, enteric rods and pseudomonads in the subgingival flora of severe adult periodontitis. Oral Microbiol Immunol 1988; 3: 47-52. [ Links ]
21. Slots J, Rams TE, Schonfeld SE. In vitro activity of chlorhexidine against enteric rods, pseudomonads and acinetobacter from human periodontitis. Oral Microbiol Immunol 1991; 6: 62-64. [ Links ]
22. Slots J, Feik D, Rams TE. In vitro antimicrobial sensitivity of enteric rods and pseudomonads from advanced adult periodontitis. Oral Microbiol Immunol 1990; 5: 298-301. [ Links ]
23. Barbosa FC, Mayer MP, Saba-Chujfi E, Cai S. Subgingival occurrence and antimicrobial susceptibility of enteric rods and pseudomonads from Brazilian periodontitis patients. Oral Microbiol Immunol 2001; 16: 306-310. [ Links ]
24. Botero JE, Contreras A, Lafaurie G, Jaramillo A, Betancourt M, Arce RM. Occurrence of periodontopathic and superinfecting bacteria in chronic and aggressive periodontitis subjects in a Colombian population. J Periodontol 2007; 78: 696-704. [ Links ]
25. Ardila CM, Fernández N, Guzmán IC. Antimicrobial susceptibility of moxifloxacin against gram-negative enteric rods from Colombian patients with chronic periodontitis. J Periodontol 2010; 81: 292-299. [ Links ]
26. Watt B, Brown FV. The in vitro activity of ciprofloxacin in combination with other agents against anaerobes of clinical interest. J Antimicrob Chemother 1986: 17: 679-680. [ Links ]
27. Ardila CM, López MA, Guzmán IC. High resistance against clindamycin, metronidazole and amoxicillin in Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans isolates of periodontal disease. Med Oral Patol Oral Cir Bucal 2010; 15(6):e947-e951. [ Links ]
28. Araujo MW, Hovey KM, Benedek JR, Grossi S, Dorn J, Wactawski-Wende J et al. Reproducibility of probing depth measurement using a constant-force electronic probe: analysis of inter- and intraexaminer variability. J Periodontol 2003; 74: 1736-1740. [ Links ]
29. Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 1999; 4: 1-6. [ Links ]
30. Möller AJ. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr 1966; 74(5) Supl: 1-380. [ Links ]
31. Slots J. Rapid identification of important periodontal microorganisms by cultivation. Oral Microbiol Immunol 1986; 1: 48-57. [ Links ]
32. Slots J, Reynolds HS. Long-wave UV light fluorescence for identification of black-pigmented Bacteroides spp. J Clin Microbiol 1982; 16: 1148-1151. [ Links ]
33. Twisk JWR. Applied multilevel analysis: a practical guide. Cambridege: Cambridge University Press; 2006. p. 196. [ Links ]
34. Guzmán IC, Grisales H, Ardila CM. Administración sistémica adjunta de moxifloxacina versus ciprofloxacina más metronidazol en el tratamiento de periodontitis crónica con presencia de bacilos entéricos Gram negativos: I. Efectos clínicos y microbiológicos. Rev Fac Odontol Univ Antioq 2011; 23(1): 92-110. [ Links ]
35. Pahkla ER, Koppel T, Naaber P, Saag M, Loivukene K. The efficacy of non-surgical and systemic antibiotic treatment on smoking and non-smoking periodontitis patients. Stomatologija 2006; 8: 116-121. [ Links ]
36. Joss A, Adler R, Lang NP. Bleeding on probing. A parameter for monitoring periodontal conditions in clinical practice. J Clin Periodontol 1994; 21: 402-408. [ Links ]
37. Darby IB, Hodge PJ, Riggio MP, Kinane DF. Clinical and microbiological effect of scaling and root planing in smoker and non-smoker chronic and aggressive periodontitis patients. J Clin Periodontol 2005; 32: 200-206. [ Links ]
38. Armitage GC, Offenbacher S. Consensus report on periodontal diseases: epidemiology and diagnosis. Ann Periodontol 1996; 1: 216-222. [ Links ]











 text in
text in