Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista Facultad de Odontología Universidad de Antioquia
versão impressa ISSN 0121-246X
Rev Fac Odontol Univ Antioq vol.25 no.1 Medellín jul./dez. 2013
ORIGINAL ARTICLES DERIVED FROM RESEARCH
COMPARISON OF THE RESISTANCE OF THREE CERAMIC SYSTEMS IN ANTERIOR FIXED PROSTHETIC SEGMENTS. A FINITE ELEMENT ANALYSIS1
Érika Alejandra Pineda Duque2; Julio César Escobar Restrepo3; Federico Latorre Correa4; Junes Abdul Villarraga Ossa5
1 Article resulting from a research project carried out by the Dentistry Biomaterials and Mechanical Design Research Groups. School of Dentistry and School of Mechanical Engineering, Universidad de Antioquia
2Dentist, Specialist in Comprehensive Dentistry of the Adult with an emphasis on Prosthodontics, School of Dentistry, Universidad de Antioquia. Email address: erikapine@hotmail.com
3Dentist, Specialist in Comprehensive Dentistry of the Adult with an emphasis on Prosthodontics, Associate Professor. School of Dentistry, Universidad de Antioquia. Email address: jcer75@yahoo.com
4 Dentist, Specialist in Comprehensive Dentistry of the Adult with an emphasis on Prosthodontics, Full Professor. School of Dentistry, Universidad de Antioquia. Email address: latorre.federico29@gmail.com
5Mechanical Engineer, Universidad Nacional de Colombia at Medellín. Msc Mechanical Engineering. Universidad Simón Bolívar, Caracas, Venezuela. Ph.D. (C) Science and Technology of Materials. Email address: junes@udea.edu.co
SUMBITTED: JUNE 8/2012-ACCEPTED: JANUARY 22/2013
Pineda ÉA, Escobar JC, Latorre F, Villarraga JA. Comparison of the resistance of three ceramic systems in anterior fixed prosthetic segments. A finite element analysis. Rev Fac Odontol Univ Antioq 2013; 25(1): 44-75.
ABSTRACT
INTRODUCTION: the purpose of this study was to evaluate stress distribution on three-unit fixed partial dentures (FPD) in the anterior region, made of three ceramic systems with connector variations. The study was performed by the finite element method. METHODS: four segments of FPD were modeled; three of them were made on ceramic systems: lithium disilicate, alumina, and zirconia, and the fourth model was of lithium disilicate with a connector of 9 mm2 in area. The modeling included three variables: elastic modulus, Poisson's ratio, and ultimate tensile strength. An initial load of 200 N was applied and increased up to 1000 N calculating von Mises, maximum tensile, compressive, and shear stresses. RESULTS AND CONCLUSIONS: all the ceramic systems showed a suitable behavior for FPDs in the anterior area; the structure's elastic modulus influences stress behavior; if it is higher, it reduces stresses in feldspar ceramics and periodontal ligament. We noted that in presence of a connector of 16 mm2 in area, the periodontal ligament received greater stresses as a compensation effect, but they significantly decreased in the structure. By reducing the connector area to 9 mm2, the stresses increased to 48%, but did not reach yield strength when subjected to loads of 1000 N, providing the system with proper margin tolerance without fracturing.
Key words: dentures, crowns, ceramics, aluminum oxide.
INTRODUCTION
In everyday practice, dentists regularly come across patients who have lost one or more teeth and therefore suffer numerous aesthetic and functional unwanted effects that affect their overall health and masticatory function.1 A possible treatment for these patients are fixed partial dentures (FPD), which are intended to restore lost teeth structure by means of all-metal, metal-ceramic, or all-ceramic restorations.2
The past two decades have witnessed the development of metal-free restorations with a mixed chemical composition: a vitreous phase which provides aesthetics, and a crystalline phase which provides resistance. Thus, depending on their chemical composition and the percentage of components, current ceramics may be classified into feldspar, lithium disilicate, alumina, and zirconia.
Lithium disilicate ceramic contains feldspar, which provides translucency; quartz, making up the crystalline phase; kaolin, which provides plasticity, and lithium disilicate which improves strength. These ceramics are provided with excellent optical properties and 75% of light transmission. Therefore, they offer optimal aesthetic characteristics for anterior area rehabilitation.3
Regarding fracture strength, this type of ceramic exceeds the threshold of 100 MPa, a figure established by ISO 6872 and registered to be between 100-300 MPa3 and a flexural strength of 350 MPa.4 These are considered as low levels, so they are indicated for veneers, single crowns and short lengths up to the premolar area, following the recommended connectors' thicknesses of 16 mm2 in areawhich is difficult to obtain in clinical normal conditions since some interocclusal space is required, and is only achieved in a few cases. It has been reported that the frequency of failure on three-unit prosthetic segments around abutment-pontic connectors is relatively high especially in thin connectors.5
FPD's deflection is inversely proportional to its width. So to prevent FPD failure, the connector must be sufficiently high and wide, and the pontic's length must not exceed certain limits.6 The primary causes of failure range from connector fracture in the case of alumina and lithium disilicate and cohesive fracture of veneering porcelain for zirconia.7
Therefore, due the risk of fracture in three-unit prosthetic segments in the anterior area, some studies recommend using ceramics such as alumina,8, 9 in which feldspathic porcelain is added significant amounts of aluminum oxide, improving the ceramic's mechanical properties with a tensile strength of 300-700 MPa and a flexural strength of 500 MPa considered to be moderate resistance, but this has the disadvantage of a large reduction in translucency, since alumina ratio reaching levels above 50% causes a significant increase in opacity greatly affecting aesthetics, which is extremely important in the anterior area.4, 9, 10
Zirconia is another ceramic of recent development; it is composed of highly sintered (95%) zirconium oxide partially stabilized with yttrium oxide (5%). It is highly tough due to its completely crystalline microstructure and is provided with a strengthening mechanism called resistant transformation. Its translucency is only 30%.3, 8 Its fracture strength is over 700 MPa and its flexural strength ranges between 1000 and 1500 MPa, but the ceramic coating with conventional feldspathic ceramic to improve its opaque appearance considerably decreases toughness. One possible explanation for this reduction in fracture strength is the formation of a compressive layer on the surface as an effect of the instrumentation, and the subsequent heat treatment and release of residual stress on the coating.1, 8
Since the literature on this subject is inconclusive, and there is no solid scientific support, professionals have made decisions about which material to use in the anterior area, based on their experiences, on lab technicians' recommendations, or solely on the materials' properties at the expense of aesthetics, since some ceramic materials do not provide optimum appearance for the anterior area.
According to the studies conducted by Reeh et al, who showed that the crown's rigidity may be 100% recovered when feldspathic porcelain is used as a substitute for enamel, most dental ceramics have a tensile strength limit greater than that of natural enamel. Therefore, materials with greater resistance seem to be unnecessary to meet the biomechanical principles. 10
The purpose of this study was therefore to evaluate stress distribution (tensile, compressive, shear, and von Mises equivalent stresses) and to compare the resistance of three ceramic systems on dental-supported fixed prosthetic segments in the anterior area under static functional loads to simulate masticatory movement behavior, using the manufacturers' specifications in their design. It was also important to evaluate their behavior when modifying the thicknesses suggested by manufacturers due to space limitations.
We hope our results provide guidelines about the specifications for these systems, or information on whether their use threatens rehabilitation, as well as information on their clinical efficacy.
The evaluation was made through 3D finite element modeling (FEM), which is widely used in medical research for its ability to reproduce the biomechanical behavior of anatomical structures through linear analysis.11, 12
METHODS
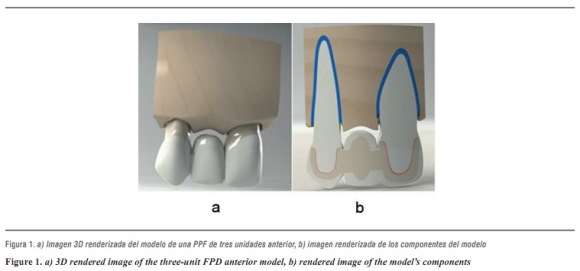
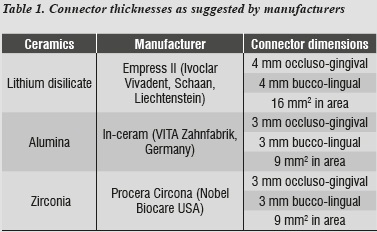
In this study, modeling was performed by means of 3-dimentional finite element analysis using CAD SolidWorks 2010, followed by SolidWork Simulation 2010 to analyze a three-unit FPD involving an upper central incisor (abutment), an upper lateral incisor (pontic) and an upper canine as abutment, with their supporting tissues: cortical bone, cancellous bone, periodontal ligament, and root. Following a standard model (figure 1), three models were designed representing the three ceramic systems under study: lithium disilicate, alumina, and zirconia, taking into account the manufacturers' thickness specifications (table 1), All the structures were attached to the stumps by a layer of resin cement and coated with feldspathic ceramic.
This area was chosen because the lateral incisor is very likely to be lost by trauma o malformations, or it may be absent due to genetic factors;13 also, this area's simple shape facilitates computer design, since the abutments usually have a relatively cylindrical pulp canal with little shape alterations.14 Another reason for this choice was the position of teeth in the arch and their inclination with respect to the Frankfort plane (tragus-orbit), being subjected to oblique forces in their longitudinal axis at a 45° angle.15
Using these three models with their respective ceramic systems to be evaluated, we noted that the requirements of Ivoclar Vivadent, Schaan, Liechtenstein16 regarding the thicknesses suggested for the connector's area in the case of lithium disilicate (table 1), required certain space conditions that are hard to achieve in real clinical situations, since a thickness of 16 mm2 in the connector's area is usually recommended, and this required a fourth model with a different thickness to that suggested by the manufacturer (9 mm2), but closer to the clinical conditions in everyday life; it is also the thickness suggested for alumina and zirconia, so one can compare the three ceramic systems with the same thicknesses.
Geometry
The central incisor and the canine were modeled individually, with a stump meeting criteria such as retention, strength and structural solidity, following the proportions needed to prepare an all-ceramic complete crown, which requires an average axial reduction of 1.8 mm. This ensured the adequate space for modeling the ceramic crown with 1.5 mm in peripheral thicknesswhile in the incisal segment it was 2 mm. We also considered the length and the cross-sectional dimension recommended for connectors in each ceramic system.16-18
All these parameters allowed achieving adequate esthetics and meeting the required thickness for material strength. The stump complied with the principles of retention and resistance required for fixed restorations, with a convergence angle of 6°, a length of 5 mm in the stump's inciso-gingival height, and dental preparation that maintained the anatomy of the tooth involved.1, 4
In the model, the upper incisor had a total length of 23.5 mm; the crown measured 10.5 mm in inciso-gingival direction, by 7.0 mm in bucco-lingual diameter and 7.4 mm in mesiodistal diameter. The root measured 13 mm long by 6.8 mm in bucco-lingual width and 7.5 mm in mesiodistal diameter. The lateral incisor's (pontic) crown's inciso-gingival height measured 9 by 6 mm in bucco-lingual diameter and 5.6 mm in mesiodistal diameter. And the modeled canine had a length of 26 mm, the inciso-gingival crown height measuring 10 by 7.5 mm in bucco-lingual diameter, and 5.5 mm in mesiodistal diameter; the modeled root was 18 mm in length by 5 mm in mesiodistal diameter and 6 mm in bucco-lingual diameter.
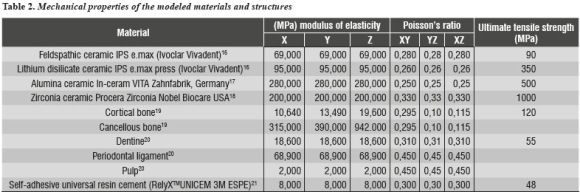
In order to calculate stress and displacement, Young's modulus of elasticity and Poisson's ratio were used for each element under study (table 2).
The mechanical properties of the elements included in the numerical model were obtained from studies reported in the literature and examined by the Biomaterials Research Group in previous studies (table 2).16-21 As a result, the model was provided with isotropic properties for the ceramic systems, dentin, periodontal ligament, and resin cement, as well as orthotropic properties for cortical and cancellous bones.
The root had a conical design in the upper central incisor and an oval shape in the upper canine.22 The periodontal ligament was designed taking into account isotropic properties (materials with similar properties in all directions); its root periphery was 0.5 mm in thickness, located 1.5 mm from the cementoenamel junction. To design alveolar bone, we included cancellous bone forming the inner maxillary mass and cortical bone surrounding both the maxilla and the alveoli. Both structures had orthotropic properties (materials with different characteristics in the x, y, and z axis). Cortical bone was 1 mm thick in the peripheral area from the basal region and 0.5 mm toward the inner region of the alveoli.23
The area of connectors was modeled according to manufacturers' specifications for each ceramic systems under study (table 1), and a fourth model was developed with lithium disilicate properties and the area recommended for alumina and zirconia (9 mm2). Equivalent comparisons were performed, and feldspathic ceramic coating was applied with a minimum thickness of 0.5 mm.16 A cement layer with a uniform thickness of 40 Μm was also modeled.24
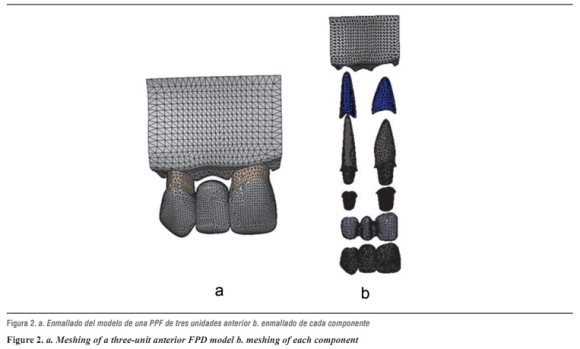
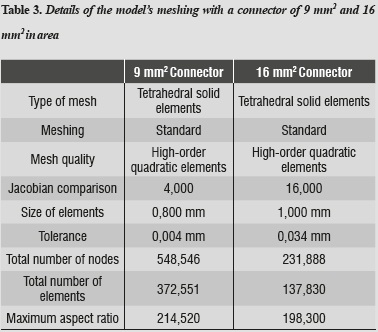
After constructing the models, a linear elastic analysis was performed using high-order tetrahedral elements (those that allow three degrees of translational and rotational freedom per node), in order to obtain a better approximation to the geometry of the parts, thereby obtaining a three-dimensional finite element mesh of the model's components (figure 2 and table 3).
To improve accuracy of the results, we used the so-called adaptive h-method, which consists in refining mesh size at the places of greatest interest to the study, in this case the area experiencing the largest displacements or stresses. Smaller elements were used in order to reduce errors and to reach acceptable values, with errors lower than 2%.
The model's tolerance, 0.004 mm for the 16 mm2 connector and 0.034 mm for the 9 mm2 connector, refer to the way elements are separated from one another into what may be considered as either contact or bonding. The quality of the mesh refers to its refinement with the addition of intermediate nodes, which improves the model's geometry.25, 26
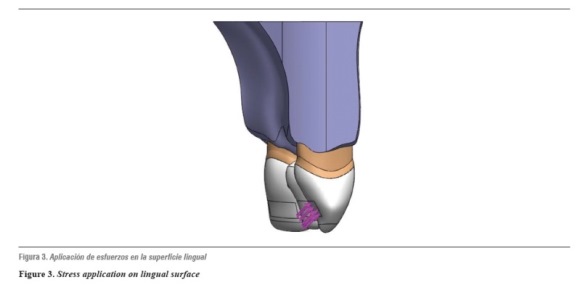
The models were loaded with a static force of initial biting reported in the literature as being 200 N,27 increased at intervals of 200 N up to 1000 N, considered in the literature as the maximum load in parafunctional activity;28 this force was applied on the middle third of the lingual crown surface at an angle of 45o for oblique stresses (figure 3).
RESULTS
By obtaining the 3D numerical modeling of an anterior FPD segment on different ceramic systems and by running simulation on a finite element software, the program provides a series of numbers so that the negative results indicate the areas of compression and the positive ones the areas of tension. A comparative analysis of von Mises, maximum tensile, compressivem, and shear stress distribution was performed on the anterior FPD sector in the ceramic systems under study, as follows:
Lithium disilicate ceramic system with a connector of 16 mm2 in area
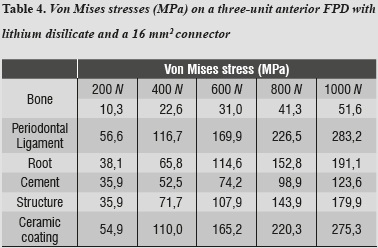
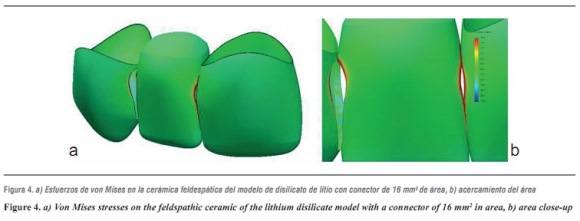
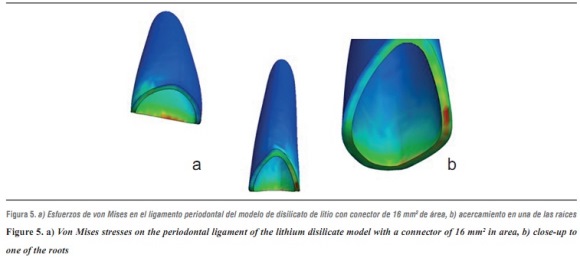
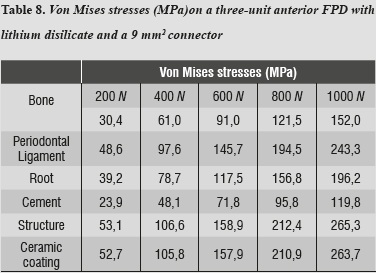
By evaluating the behavior of von Mises stresses, we observed a similar behavior when increasing loads; the structures subjected to the highest stresses were periodontal ligament and ceramic coating, especially at the area of connectors. Also, by increasing the load up to 1000 N, no fault values are obtained on the material forming the cap, which is responsible for FPD's structural solidity. The ceramic coating reached its ultimate stress under a load of 400 N (table 4, figures 4a, b and figures 5a, b).
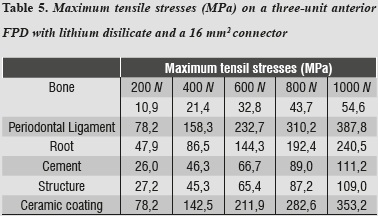
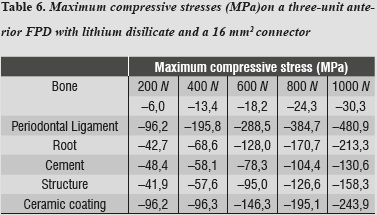
Tables 5 and 6 show that the structure that received maximum tensile stresses was the periodontal ligament at the palatal surface, and maximum compressive stress occurred on the labial surface, followed by the ceramic coating at the area of connectors, the values being very similar in both structures.
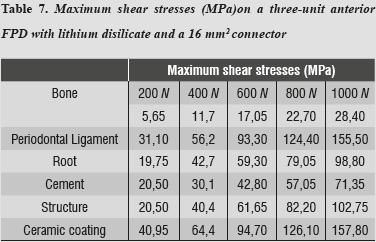
Regarding shear stresses, the most affected structures were ceramic coating and periodontal ligament; the lowest stresses occurred on the cement (table 7).
Lithium disilicate ceramic system with a connector of 9 mm2 in area
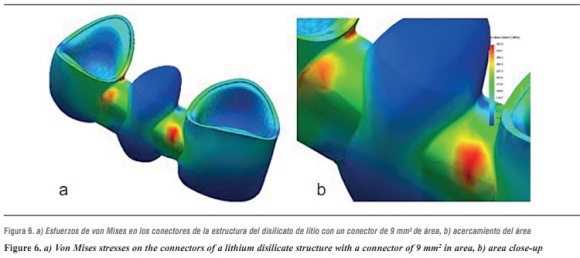
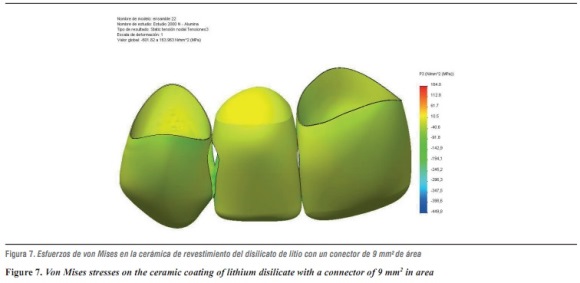
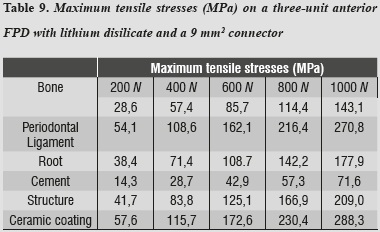
In terms of von Mises stresses on the lithium disilicate ceramic system with reduced thickness for the connector with 9 mm2 in area, we observed that the behavior is similar when increasing loads. The parts subjected to the highest stresses were the ceramic structure followed by the ceramic coating at the area of connectors, with similar values. By increasing the load up to 1000 N, no fault values were achieved on the material forming the cap, which is responsible for FPD's structural solidity; the ceramic coating reached its yield strength under a load of 400 N (table 8 and figures 6a, b, and 7).
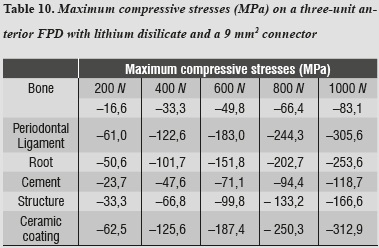
By evaluating maximum tensile and maximum compressive stresses, one may observe that ceramic coating was the one that received the highest values of both types of stress (tables 9 and 10).
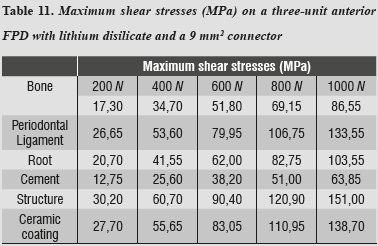
Maximum shear stresses are higher on the ceramic structure; it may also be observed that the cement receives the lowest shear stresses (table 11).
Alumina ceramic system with a connector of 9 mm2 in area
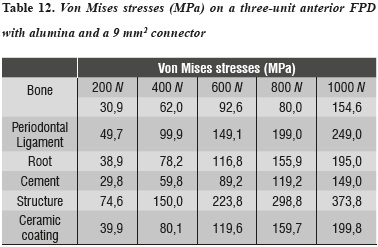
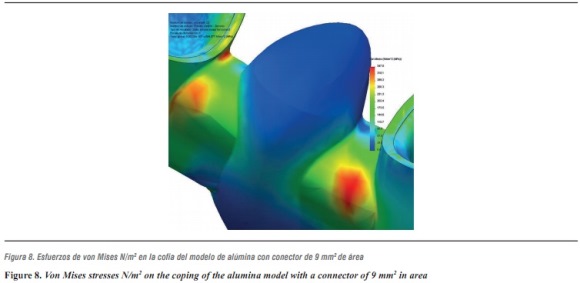
For the alumina ceramic system with a connector of 9 mm2 in area, the highest von Mises stresses occurred on the ceramic structure at the area of connectors (table 12 and figure 8).
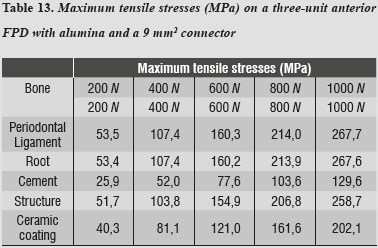
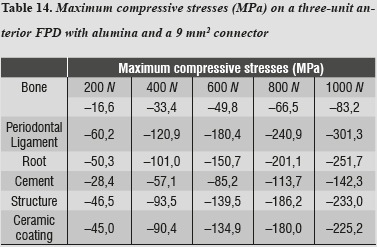
For both maximum tensile and maximum compressive stresses, the periodontal ligament was the one subjected to the highest values (tables 13 and 14).
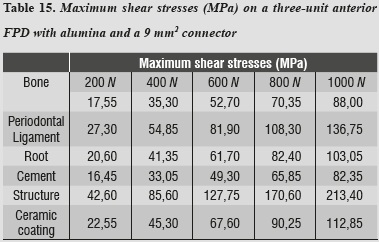
Maximum shear stresses occurred on the ceramic structure; one can also observe that the cement received very low stresses (table 15).
Zirconia ceramic system with a connector of 9 mm2 in area
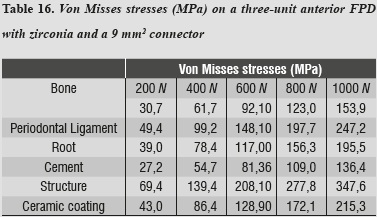
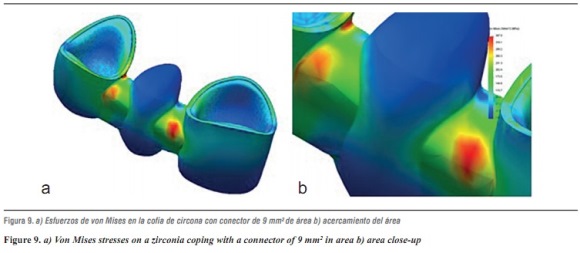
For the zirconia ceramic system, the thicknesses suggested by Nobel Biocare USA are those of a connector with an area of 9 mm2; as for the alumina model, the highest von Mises stresses shown by the system occurred on the ceramic structure at the area of connectors (table 16 and figures 9a and b).
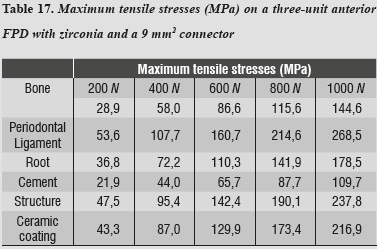
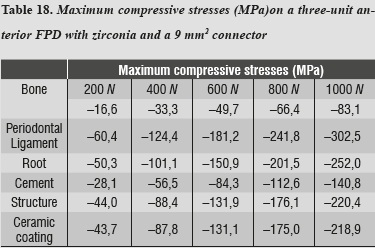
In evaluating the maximum tensile and maximum compressive stress levels in this model, we observed that periodontal ligament was the one subjected to the highest levels of both type of stresses (tables 17 and 18).
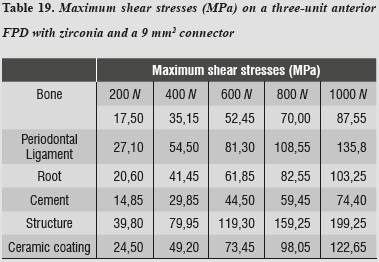
As in the previous model, maximum shear stresses occur on the ceramic structure; it may also be noted that cement receives very low stresses (table 19).
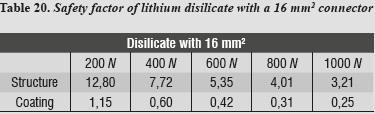
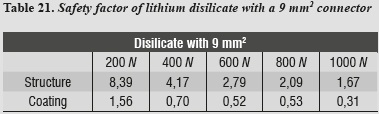
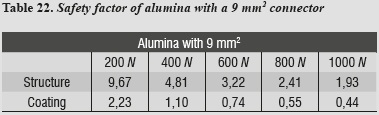
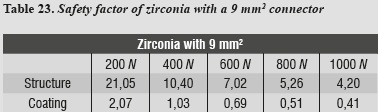
A safety factor was also generated (tables 20 - 23) allowing us to observe the working threshold in each ceramic system under study; this shows that when using a material with higher elastic modulus (alumina), the ceramics coating exhibits a greater safety factor, resisting load values higher than those occurring when using a material with a lower elastic modulus as in the case of lithium disilicate.
This safety factor is obtained by dividing the value of the target material's ultimate tensile strength into the maximum tensile stress value under each load. Failure occurred when values were < 1; the higher the results the greater the safety factor, efficiently moving away from failure values.
DISCUSSION
Because scientific evidence is scarce and inconclusive, the decision on which material to use in the anterior area is usually made following manufacturers` recommendations, solely based on the material's resistance properties16-18 at the expense of esthetics, since the toughest ceramic materials do not provide optimum visual properties for this area.
Therefore, this study used 3D finite element modeling to evaluate stress distribution comparing the resistance of three ceramic systems (lithium disilicate, alumina, and zirconia) in dental-supported fixed prosthetic segments in the anterior area, using manufacturers' specifications and assessing their behavior when the suggested thicknesses were modified to simulate space limitations.
This study considered the maximum masticatory force to which these materials were subjected. Besides individual anatomical and physiological characteristics, it also considered bite force in different areas of the oral cavity. In previous studies, the mean values of maximum masticatory force vary from 216-847 N.27-29 The values reported for the anterior area are lower and are in the range of 108-299 N.30, 31 Based on these scientific reports, we decided to supply an initial load of 200 N and a maximum load 1000 Na limit normally used as a range of security required for a positive clinical outcome of all-ceramic FPDs.8 It is important to note that, in the oral environment, the loads applied to dental restorations are cyclic in nature. Therefore, the cyclic loads may more accurately simulate the masticatory forces of static charges used in this research study, and therefore become a limitation of it.
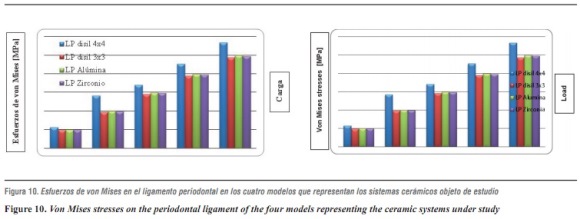
In evaluating stress distribution through the 3D numerical model that represents the lithium disilicate ceramic system with a connector of 16 mm2 in area as suggested by the manufacturer, one may observe that the behavior is similar when loads are increased. The structure subjected to the highest von Mises stresses was periodontal ligament; by having a structure with increased rigidity containing wider connectors, the ligament allowed significant root displacements (figure 10), i.e., it functions as an interface or elastic foundation, which reduces or dampens the stresses on the other elements. Thus, this element helps to protect the entire system. According to Campbell and Sozio,32 by modeling the periodontal ligament we are taking into consideration abutment teeth mobility when evaluating FPD ceramic systems, since under a load or an occlusal stress teeth experience deflection by compensation of Sharpey's fibers, and thus it is possible to extrapolate the data to clinical situations.
The second structure in receiving higher von Mises, maximum tensile and compressive stresses was ceramic coating, especially in the area of connectors; this is probably due to the fact that this is the element with the lowest modulus of elasticity, and the one that reached its yield strength under loads of 400 to 600 MPa in all the models. This finding is similar to that by Kelly et al,33 in which 70 to 78% of the samples (in vitro and in vivo) showed fracture lines starting at the ceramic coating or at its interface with the structure, indicating that ceramic coating is a structure that experiences high tensile stresses, and is therefore an important source of structural failure. For this reason detachment or fracture is the most common complication reported, especially when using a material with a low modulus of elasticity for the supporting structure.7 Tinschert et al8 recommend using a very thin layer or no ceramic coating at all on the connector's gingival wall, as this allows to maximize the resistance provided by the material of the structure. However, they explain that ceramic coating has no independent behavior but is stably attached to the structure, and their study shows that ceramic coating obtains its strength from the structure's material, which could generate resistance levels higher than the one reported in our study.
As loads were increased up to 1000 N, no other material reached failure values; maximum shear stresses were present in the structure and the lowest ones occurred on the cement, ensuring adequate structure retention.
Our study allows us to conclude that lithium disilicate in FPD in the anterior area with connectors of 16 mm2 being a suitable material for rehabilitation in these cases. This result agrees with the study by Tinschert et al,8 who recommend using disilicate up to the premolar region, following manufacturer's instructions. Also, in a clinical study of 60 three-unit FPD made of lithium disilicate, Sorensen et al34 identified a failure rate of 7% in a period of 6 to 12 months, when four FPD failed due to structural fractures. Three of these failures occurred in the premolar area, while only one was observed in the anterior area. Thus, these authors also suggest the use of lithium disilicate for FPD up to the premolar area with a connector height no less than 5 mm longprovided that it may be obtained; although it should be noted that their study included a very small sample and in a short evaluation period.
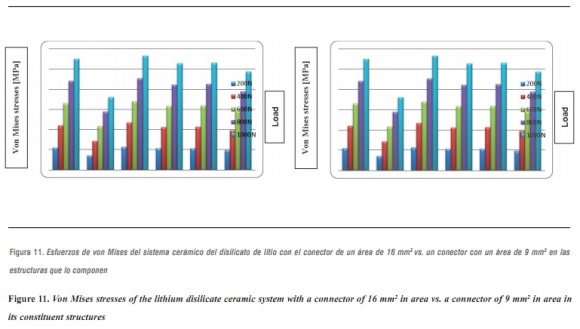
The above results were compared with the model that represents the lithium disilicate ceramic system with a connector of 9 mm2 in area. This is a clinical condition that occurs in cases of limited space in which the von Mises stresses are increased in 48%, so that the structure is the one that supports the highest stresses, followed by the ceramic coating in the area of connectors, with very similar values (figure 11). However, by increasing loads on the system up to 1000 N it does not reach levels exceeding yield stress for the structure's material, suggesting that, by reducing the area of the connector, no FPD fracture occurs, favorably supporting loads up to 1000 N. This result is similar to the one by Kamposiora et al3535 who, by using a 2D finite element analysis for FPD from the mandibular first premolar to the first molar, made of various materials: alloys with high type III gold content, Dicor Ceram, and In-Ceram, compared connectors of different heights (3 mm vs. 4 mm), finding out that the resulting von Mises stresses were concentrated on the connector. The stresses were lower for 4 mm connectors in 40 to 50%. Thus, by increasing connector height they dramatically reduced the stresses. Barreira et al,36 in their 2D finite elements study in the posterior area, compared stress distribution in models with the properties of metal-ceramic FPDs and all-ceramics FPDs, and found out that the maximum tensile stress always occurs at the connector, so they considered this point as the one of greatest risk for the prosthesis as it presents the biggest fracture potential, and therefore suggest optimizing the connector`s dimension. But in both studies the numerical models were located in the posterior area, differing with our modeling methodology. After an extensive search for items resembling the methodology of our research, we found some similarities with Pospiech et al,37 who carried out a finite element analysis on a 3D model of an FPD in the anterior area, with an alumina structure under a load of 250 N, and evaluated stress distribution in the connector area, concluding that a connector of 3 mm in height results in stresses above 534 MPa unlike a connector with greater height (4 mm), which reduces the stresses to 143 MPa, so they recommend connectors height of at least 4 mm for anterior restorations; it is important to note that the ceramic material under evaluation in their study was alumina.
Lithium disilicate FPDs with lower survival rates7 experienced structure fractures as the primary cause of failure; this occurred in cases where the connector's dimensions did followed the technical recommendations of 16 mm2. This information differs from that obtained in this study.
In addition to the data obtained by reducing connector area, we found out that maximum tensile and maximum compressive stresses occurred on the ceramic coating, and maximum shear stresses occurred on the structure, requiring the same structures of the previous model.
The results obtained with lithium disilicate were compared with alumina and zirconia ceramic systems since it has been reported that these materials perform better in terms of fracture strength in anterior and posterior FPDs.7-9, 34 Therefore, the von Mises stresses of lithium disilicate with a 16 mm2 connector were higher by 108% compared to the 3D mathematical model of a previous FPD designed according to the properties of the alumina ceramic system using the thickness suggested by VITA Zahnfabrik, Germany of a connector with 9 mm2 in area, where stresses were significantly lower. Its strength characteristics are due to its high content of aluminum oxide and to the infiltration with low viscosity molten glass, known as lanthanum glass, which eliminates porosity and limits the potential sites for crevice propagation.7
Kamposiora35 used a 2D finite element model to analyze stress distribution on an FPD made of In-Ceram alumina, an alloy with high concentrations of gold and Dicor ceramic, and found out that the stresses were much lower on the In-Ceram alumina FPD concluding that this appears to be the most successful type of restoration out of the ones they evaluated. By using a 3D finite element model of a three-unit FDP in the anterior area, made of alumina under a load of 250 N and angles of 45 and 60°, Pospiech37 found that this types of FPD are recommended if the connector's height reaches at least 4 mm.
This result is consistent with clinical studies reported by Sorensen et al,34 where 61 three-unit FPDs were evaluated and none failed in the anterior area; 35% of failure rate was found in the posterior area after 3 years. These failures were due to the propagation of fault lines in the connector area. This finding was similar to that of our study; the highest von Mises stresses occurred on the ceramic structure at the area of connectors.
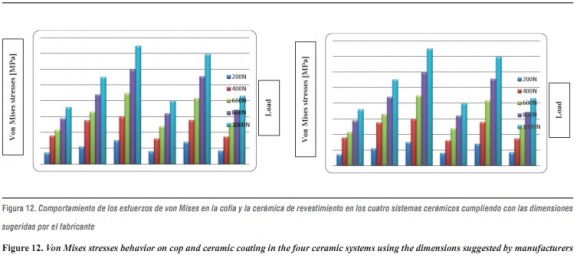
Comparing stress distribution, we observed that by increasing the elastic modulus of the material forming the cap (in this study, alumina is the material with the highest elastic modulus), the stresses on the feldspathic ceramic decrease showing less deformation and less tendency to fracture (figure 12). This agrees with other reports that suggest that the material's resistance is more important than ceramic coating because the structure's material better supports flexural load while functioning.38
By using the 3D alumina model to asses maximum tensile and maximum compressive stresses, we observed that periodontal ligament was the one subjected to the highest values, showing a similar behavior to that of lithium disilicate with a 16 mm2 connector. This might be explained by a compensation phenomenon of the periodontal ligament when using rigid materials for restoration. By increasing loads on the system up to 1000 N no failure values were reached for any other material; maximum shear stresses occurred on the structure and the lowest occurred on cement, ensuring adequate structure retention.
When comparing the von Mises stresses of lithium disilicate with 16 mm2 connector and the stresses obtained in the 3D mathematical model of an anterior FPD designed taking into account the properties of zirconia ceramic system using the thickness suggested by Nobel Biocare USA for a 9 mm2 connector, we noted that the highest von Misses stresses occurred on the lithium disilicate model by 93%. Just as in the alumina model, the system's highest von Mises stresses occurred on the ceramic structure at the area of connectors. The maximum load of 1000 N was far from reaching yield strength in this material, as it has high elastic modulus, besides being partially stabilized which yttrium, providing it excellent mechanical properties, as well as dimensional and chemical stability. Restorations performed by instrumenting fully sintered blocks have shown a higher amount of monoclinic zirconia, which may be associated with microfractures the primary cause of failure.39 All-ceramic systems based on lithium disilicate structures show a high rate of detachment (9.8%) after an observation period of 52 months, as reported in the literature. In contrast, clinical studies of In-ceram alumina ceramics report no complications associated with the coating material.40 Furthermore, the clinical studies that evaluate zirconia ceramic system in the anterior area report in this sense a low incidence of defects in the coating material. These data are identical to those found in our study, in which we concluded that to increase the elastic modulus of the material forming the cap, the stresses on feldspathic ceramics are reduced.
There is currently very little evidence of zirconia restorations in the anterior area; most are focused on evaluating its behavior on posterior FPDs, showing the limitations of this ceramic system to provide favorable optical and aesthetic properties in the anterior area. Information about zirconia in the anterior area was only obtained in the article by Edelhoff et al,40 who evaluated the clinical behavior of 21 three- to six-unit FPDs in the anterior and posterior area during 3 years; the structures were developed with the CAD/CAM, and no fractures were observed on the structures. However, detachment of the ceramic coating occurred in two anterior area cases. They conclude that all-ceramic zirconia FPDs showed a satisfactory clinical behavior with a success rate of 92%.
In this model, by evaluating maximum tensile and maximum compressive stresses, we observed that periodontal ligament was the one subjected to the highest levels of both types of stress, and as in the case of alumina, this phenomenon is associated with the system's rigidity which requires greater compensation displacement of the periodontal ligament.
Currently, there is not enough evidence of ceramic fracture analysis. In 2012, Anuvisace stated that the specific etiology of ceramic fractures is unknown, so it is expected to provide further information leading to preventive measures for structure design and thermal processing methods.41
In 2010, Quinn presented Weibull's analysis as a tool with indications and limitations, but it is important to evaluate the possibility of fracture in ceramic systems.42
It is important to have information on stress distribution, but according to Möllers et al there are few studies examining connectors. In their study, Möllers et al found a connector behavior similar to the one in our study.43
In a 2003 study using FEM and Weibull analysis, Fischer et al argue that the connector's design is critical to the restoration, just as we suggest in our study. Also, the predictive value of FEM and the Weibull statistical tool demonstrate that zirconia and lithium disilicate are safe to use. In our study, the three ceramic systems have a high safety factor, validating Fischer's analysis.44
Consequently, the recommendation of which system to choose must be accompanied by precautions related to the connector, considering that there are some variables that our study and others have failed to fully address, such as material fatigue and factors closely connected to the material's manufacturing process itself.
Final decision and behavior are therefore determined by multiple factors, including important considerations such as cost, adherence and the esthetics that can be achieved with each of these restorations.
We did not find studies with approach and methodology similar to the ones used in our study, limiting equivalent comparisons with other research. This study is a guide for future studies on current ceramic systems.
CONCLUSIONS
Considering the limitations of this study, the finite element analysis indicated:
- None of the evaluated ceramic systems reached fracture limit under loads of 1000 N, so we can conclude that all these ceramic systems present adequate stress distribution and are suitable for FPD in the anterior area; the structure's elastic modulus influences stress behavior, and if higher it decreases the stresses on feldspathic ceramics and periodontal ligament.
- Concerning connectors' thickness, it was shown that in presence of an area of 16 mm2, the periodontal ligament received greater stresses as a compensation effect, but the stresses significantly decreased on the coping. By reducing the area of the connectors to 9 mm2, the stresses increased by 48%, but did not reach the fracture limit of 350 MPa when subjected to loads of 1000 Nhardly found in the stomatognathic system, providing the lithium disilicate system with adequate tolerance without reaching the point of risk.
REFERENCES
1. Rosenstiel S, Land M, Fujimoto J. All ceramic restorations. En: Contemporary fixed prosthodontics. 4.ª ed. Barcelona: Elsevier; 2009. p. 774-804. [ Links ]
2. Shillingburg HT, Hobo S, Whitsett L, Jacobi R, Brackett S. Fixed partial denture configurations. En: Fundamentals of fixed prosthodontics. 3.ª ed. Chicago: Quintessence; 1997. p. 105-118. [ Links ]
3. Raigrodski AJ. Contemporary all-ceramic fixed partial dentures: a review. Dent Clin North Am 2004; 48(2): 531- 544. [ Links ]
4. Massironi D, Pascetta R, Romeo G. Using ceramic in prosthetic restoration. En: Precision in dental esthetics: clinical and laboratory procedures. Milán: Quintessence; 2007. p. 342-373. [ Links ]
5. Goodacre CJ, Bernal G, Rungcharassaeng K, Kan JY. Clinical complications in fixed prosthodontics. J Prosthet Dent 2003; 90(1): 31-41. [ Links ]
6. Raigrodski AJ, Chiche GJ. The safety and efficacy of anterior ceramic fixed partial dentures: A review of the literature. J Prosthet Dent 2001; 86(5): 520-525 [ Links ]
7. Conrad HJ, Seong WJ, Pesun IJ. Current ceramic materials and systems with clinical recommendations: a systematic review. J Prosthet Dent 2007; 98(5): 389-404. [ Links ]
8. Tinschert O, Natt G, Mautsch W, Augthun M, Spiekermann H. Fracture resistance of lithium disilicate, alumina, and zircona-based three-unit fixed partial dentures: a laboratory study. Int J Prosthodont 2001; 14 (3): 231-238. [ Links ]
9. Wassermann A, Kaiser M, Strub JR. Clinical long-term results of VITA in-ceram classic crowns and fixed partial dentures: a systematic literature review. Int J Prosthodont 2006; 19(4): 355-363. [ Links ]
10. Pascal M, Urs B. Understanding the intact tooth and the biomimetic principle. En: bonded porcelain restorations in the anterior dentition: a biomimetic approach. Barcelona: Quintessence; 2004. p. 23-56. [ Links ]
11. Asmussen E, Peutzfeldt A, Sahafi A. Finite elements analysis of stresses in endodontically treated, dowel-restored teeth. J Prosthet Dent 2005; 94 (4): 321-329. [ Links ]
12. Srirekha A, Bashetty K. Infinite to finite: an overview of finite element analysis. Indian J Dent Res 2010; 21(3): 425-432. [ Links ]
13. Graber TM, Vanarsdall RL, Katherine WL. Diagnosis and treatment planning in orthodontics. In: Orthodontics: current principles and techniques. 3.ª ed. Philadelfia: Mosby; 2000. p. 28. [ Links ]
14. JOE Editorial Board. Root canal anatomy: an online study guide. J Endod 2008; 34(5 Suppl): e7-e16. [ Links ]
15. Moyer R, Bookstein F, Hunter WS. Analysis of the craniofacial skeleton: cefalometrics. En: Handbook of Orthodontics. 4.ª ed. Ann Arbor: Year book Medical Publishers; 1988. p. 247-301. [ Links ]
16. Ivoclar Vivadent. Ficha técnica IPS e.max Press [Internet]. [Consultado 2011 mayo 05]. Disponible en: http://www. ivoclarvivadent.com/en/.../ips-emax.../ips-emax-press [ Links ]
17. VITA. Ficha técnica VITA In-ceram alúmina [internet]. [Consultado 2011 mayo 05]. Disponible en:http://www. vita-zahnfabrik.com-resourcesvita-shop-en-en-3053408. pdf [ Links ]
18. Nobel Biocare. Ficha técnica Procera Circona [Internet]. [Consultado 2011 mayo 02]. Disponible en: http://www. nobelbiocare.com/.../Pre-prog_Brazil_WEB_ESL_C1.pdf [ Links ]
19. Rho JY, Ashman RB, Turner CH. Young's modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J Biomech 1993; 26: 111-119. [ Links ]
20. Reinhardt RA, Krejci RF, Pao YC, Stannard JG. Dentin stress in post-reconstructed teeth with diminishing bone support. J Dent Res 1983; 62(9): 1002-1008. [ Links ]
21. 3M ESPE. Ficha técnica RelyX™Unicem. Aplicap™/ Maxicap™ [Internet]. [Consultado 2011 abril 18]. Disponible en:http://www.ddsltdlab.com/pdf/Cementation- Techniques.pdf [ Links ]
22. Woelfel JB. Anterior Teeth anatomy. In: Dental anatomy: its relevance to dentistry. 5.ª ed. Philadelphia: Williams & Wilkins; 1997. p. 119-170. [ Links ]
23. Roomed S, Fok S, Wilson N. A comparison of 2D and 3D finite element analysis of a restored tooth. J Oral Rehabil 2006; 33: 209-215. [ Links ]
24. Carter SM, Wilson PR. The effect of die spacing on crown retention. Int J Prosthodont 1996; 9(1): 21-29. [ Links ]
25. Mottram JT, Shaw CT. Finite element method. In: Mottram JT. Using finite elements in mechanical design. New York: McGraw-Hill; 1996. p. 13-14. [ Links ]
26. Reddy JN. Boundary element method. In: Reddy JN. An introduction to the finite element method. 3.ª ed. New York: McGraw-Hill; 2005. p. 442-43. [ Links ]
27. Helkimo E, Carlsson GE, Helkimo M. Bite force and state of dentition. Acta Odontol Scand 1977; 35(6): 297-303. [ Links ]
28. Raadsheer MC, Van Eijden TM, Van Ginkel FC, Prahl-Andersen B. Contribution of jaw muscle size and craniofacial morphology to human bite magnitude. J Dent Res 1999; 78(1): 31-42. [ Links ]
29. Waltimo A, Kemppainen P, Könönen M. Maximal contraction force and endurance of human jaw-closing muscles in isometric clenching. Scand J Dent Res 1993; 101: 416- 421. [ Links ]
30. Linderholm H, Wennström A. Isometric bite force and its relation to general muscle force and body build. Acta Odontol Scand 1970; 28: 679-689. [ Links ]
31. Ringqvist M. Isometric bite forces and its relation to dimensions of facial skeleton. Acta Odontol Scand 1973; 31: 35-42. [ Links ].
32. Campbell SD, Sozio RB. Evaluation of the fit and strength of an all-ceramic fixed partial denture. J Prosthet Dent 1988; 59: 301-306. [ Links ]
33. Kelly JR, Teskl JA, Sorensen JA. Failure of all-ceramic fixed partial dentures in vitro and in vivo: analysis and modeling. J Dent Res 1995; 74(6): 1253-1258. [ Links ]
34. Sorensen JA, Kang S-K, Torres TJ, Knode H. In-Ceram fixed partial dentures: Three-year clinical trial results. J Calif Dent Assoc 1998; 26: 207-214. [ Links ]
35. Kamposiora P, Papavasiliou G, Bayne SC, Fellon DA. Stress concentration in all-ceramic posterior fixed partial dentures. Quintessence Int 1996; 27: 701-706. [ Links ]
36. Barreira A, Pereira LC, Da Cunha A, Pereira F. The in- ?uence of the loading mode on the stress distribution on the connector region of metal-ceramic and all-ceramic fixed partial denture. Artif Organs 2008; 32(4): 283-291. [ Links ]
37. Pospiech P, Rammelsberg P, Goldhofer G, Gernet W. All-ceramic resin-bonded bridges. A 3-dimensional finite- element analysis study. Eur J Oral Sci 1996; 104: 390- 395. [ Links ]
38. Pilathadka S, Vahalová D. Contemporary all-ceramic materials part-1. Acta Médica 2007; 50(2): 101-104. [ Links ]
39. Manicone PF, Iommetti R, Raffaelli L. An overview of circona ceramics: basis proprieties and clinical applications. J Dent 2007; 35(11): 819-826. [ Links ]
40. Edelhoff D, Beuer F, Weber V, Johnen C. HIP circona fixed partial dentures-Clinical results after 3 years of clinical service. Quintessence Int 2008; 39: 459-471. [ Links ]
41. Anuvisace K. Standardizing failure, success, and survival decisions in clinical studies of ceramic and metal-ceramic fixed dental prostheses. Dent Mater 2012; 28(1): 102-111. [ Links ]
42. Quinn J, Quinn G. A practical and systematic review of Weibull statistics for reporting strengths of dental materials. Dent Mater 2010; 26(2): 135-147. [ Links ]
43. Möllers K, Pätzold W, Parkot D, Kirsten A, Güth JF, Edelhoff D et al. Influence of connector design and material composition and veneering on the stress distribution of all-ceramic fixed dental prostheses: a finite element study. Dent Mater 2011; 27(8): e171-e175. [ Links ]
44. Fischer H, Weber M, Marx R. Lifetime prediction of all-ceramic bridges by computational methods. J Dent Res 2003; 82: 238-242. [ Links ]











 texto em
texto em