Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Odontología Universidad de Antioquia
Print version ISSN 0121-246X
Rev Fac Odontol Univ Antioq vol.25 no.1 Medellín July/Dec. 2013
ORIGINAL ARTICLES DERIVED FROM RESEARCH
INFLUENCE OF POST-BLEACHING TIME ON A COMPOSITE RESIN BOND STRENGTH TO ENAMEL1
Paula Alejandra Baldión Elorza2
1 Article derived from a research project to opt to the category of Associate Professor. Grupo de Investigación en Materiales Dentales (GRIMAD). With institutional support from Universidad Nacional de Colombia School of Dentistry
2Dentist, Specialist in Oral Rehabilitation, Faculty of Dentistry, Universidad Nacional de Colombia. Professor, Department of Oral Health, School of Dentistry, Universidad Nacional de Colombia at Bogotá. E-mail address: pabaldione@unal.edu.co
SUMBITTED: OCTOBER 23/2012-ACCEPTED: FEBRUARY 5/2013
Baldión PA. Influence of post-bleaching time on the adhesion of a composite resin to enamel. Rev Fac Odontol Univ Antioq 2013; 25(1) 92-116.
ABSTRACT
INTRODUCTION: despite the aesthetic advantages of tooth whitening, it has been shown to alter the adhesion of composite resins to enamel—a process determined by the residual concentration of oxygen-free radicals on the enamel's surface and subsurface due to degradation of the bleaching agents' hydrogen peroxide—. The goal of this study was to evaluate the effect of hydrogen peroxide on a composite resin's bond strength to enamel in different time intervals after whitening. METHODS: 90 human premolars were selected and sorted out in 6 groups: the control group, which was subjected to the adhesive technique only, and five study groups treated with the bleaching agent and each of them being later subjected to the adhesive technique at different time intervals after whitening (0, 1, 7, 14, and 28 days), in order to determine the shear bond strength of the resin adhered to enamel. The obtained data were analyzed by means of the ANOVA test with the F statistical test and Fisher & Duncan multiple comparisons, using p values of < 0.05 as statistically significant differences. RESULTS: The groups undergoing bleaching from 0 to 28 days presented adhesion values statistically lower than those of the control group. CONCLUSIONS: hydrogen peroxide reduces the values of adhesion to enamel. Post-bleaching time is crucial to recover the composite resin bonding strength to dental structure.
Key words: enamel, dental bonding, tooth whitening, composite resin, hydrogen peroxide.
INTRODUCTION
The effects of bleaching agents on both dental tissues and composite resins' bond strength after whitening have been described in numerous studies including the ones by Dishman et al,1 van der Vyver et al,2 Homewood et al,3 and Cappeletto et al.4 However, the ways to minimize or reverse undesirable effects are still under study, and there is currently much concern in determining the best way to perform aesthetically successful clinical procedures while at the same time providing biological safety, reducing side effects during and after treating oral tissues and dental materials.5, 6 For this reason, more studies are required to substantiate alternative clinical protocols to improve the prognosis of adhesive restorations after whitening.
Cobankara et al7 and Pinto et al8 have reported alterations produced by applying bleaching agents on dental structures such as microstructural changes and tooth enamel roughness, evaluated by electron microscopy. Gotz et al9 showed changes in the enamel's chemical composition due to mineral content loss, as well as alterations in dental fluorescence and dehydration.
Claus10 and Moreira de Freitas et al11 reported decreased surface hardness and wear resistance of the structure of both enamel and dentin. These drawbacks have been attributed to the bleaching agents' mechanism of action, which is based on degradation of hydrogen peroxide—a highly reactive, unstable substance capable of slowly breaking down into oxygen and water with heat release—. Decomposition rates may increase in the presence of catalysts, triggering a redox reaction or an oxidation- reduction reaction consisting of electron transfers between the reacting substances leading to a change in their oxidation states in relation to the products.12
Hydrogen peroxide acts as an oxidizing agent that tends to capture electrons from the environment and is therefore reduced, while pigment molecules of long molecular chains that are embedded in a given structure function as a reducing agent that provides the environment being oxidized with electrons of its chemical structure, breaking the single and double bonds of extended conjugated chains. This chemical process results in the transformation from dark pigments or chromophores, which often include heteroatoms, carbonyl and phenyl rings,13 to structures of unsaturated linear molecular chains weakly pigmented that later become nonpigmented hydrophilic structures that facilitate the passage of light through the enamel structure until reaching the so-called "saturation point",14 defined as the complete fractionation of linear chains of pigments.
The released gaseous oxygen molecules have the ability to penetrate the enamel through its natural permeability canals, such as prism sheaths, the intercrystalline matrix, Retzius striae, enamel lamellae, and spindle bodies or adamantine spindles,15 or through porous areas produced by demineralization associated with the low pH of certain bleaching agents.16
Oxygen radicals are very small molecules easily penetrable and they are highly reactive due to the presence of a layer of unpaired valence electrons; their reactivity depends on the reaction conditions, including temperature, pH, light, and the presence of transition metals.13 Hydrogen peroxide can produce different types of reactive oxygen species (ROS); under alkaline conditions, hydrogen peroxide works via the hydroperoxyl anion (HO2-) and under different pH conditions certain free radicals are formed, such as the superoxide anion (O2-) and the hydroxyl anion (OH-),13, 17 which may result from either the Fenton reaction, which involves the presence of metal ions and subspecies, or the Haber-Weiss reaction from peroxide hydrogen and superoxide, thus producing radicals (OH-).18
Oxidative stress has been defined as a phenomenon that triggers significant structure changes due to exposure of vital tissues to various sources that may alter the necessary balance between the amount of reactive oxygen species and prooxidant substances or factors and antioxidant mechanisms (such as peroxidase)—responsible for eliminating such chemical species—.19 In the case of whitening, this mechanism is not properly regulated due to an excessive increase in the production of reactive oxygen species as a result of using carbamide peroxide or hydrogen and sodium perborate.20
Mc Guckin et al20 and Titley et al21 have reported that the presence of residual peroxide or oxygen released by bleaching agents, besides having a potential deleterious influence on oral tissues, may inhibit the polymerization process of both adhesive systems and composite resins by interrupting the formation of the three-dimensional net of the long polymer chain of methacrylate-based resins, reducing their conversion degree. As a consequence, a bond strength decrease has been reported, which leads several authors, including van der Vyver et al,2 Cappeletto et al,4 Carvalli et al,22 Rotstein et al,23 Shinora et al24 and Sung et al,25 to suggest that restorative procedures should be postponed for two to four weeks after whitening, since the reduction of bonding strength has proven to be transient.24
Dishman et al1 reported a decrease in adhesion to dentin after applying 25% hydrogen peroxide—a result they attribute to inadequate formation of the adhesive interdigitations at the resin-enamel interface.
Shinohara et al24 showed that the values of shear bond strength after whitening are time dependent, so they recommend waiting two weeks after teeth whitening to perform satisfactory adhesive restorations on enamel and dentin; these findings are similar to the ones reported by van der Vyver et al,2 who describe the same phenomenon of decreased adhesive strength values when carbamide peroxide is applied and activated by light.
Sung et al25 concluded that adhesive bond strength to dentin decreases with the prior application of a bleaching agent containing 10% carbamide peroxide, as they found out a correlation with the type of solvent used as a component of adhesive systems; in fact, they reported greater bonding loss in acetone- based adhesives compared to alcohol-based adhesives.
Furthermore, Cadenaro et al26 suggest that adhesive systems applied on the dentin surface after whitening present low conversion degrees due to the polymerization inhibition produced by the residual oxygen covering the tooth structure, and that a fourteen-day period, or prolonged polymerization, may improve the conversion degree of dentin adhesives.
On the other hand, authors such as Ben-Amar et al27 and Josey et al28 consider this effect as the result of chemical composition changes of the enamel structure after whitening.29
The purpose of this study was to evaluate the effect of bleaching with hydrogen peroxide at a high concentration (38%) on an ethanol-based adhesive system and a nanohybrid composite resin shear bond strength to dentin at different time intervals (0, 1, 7, 14, and 28 days) after teeth whitening.
METHODS
For this in vitro quantitative experimental study, the researchers selected 90 human premolars recently extracted for orthodontic reasons and stored for no more than three months after extraction.
All the teeth were selected according to these inclusion criteria: fully developed roots and absence of the following conditions: cavities or enamel developmental defects, previous restorations, fracture lines or full enamel fractures, and dentin exposed due to attrition or superficial lesions. This study took into account ethical considerations following both the Declaration of Helsinki30 and the Ministry of Health Resolution No. 8430 of 1993,31 according to which this one is classified as a minimal risk research. A form was filled and signed by each patient giving their consent to donate their teeth specifically for this study, which was approved by the Ethics Committee of Universidad Nacional de Colombia School of Dentistry.
Soft tissue remains on the teeth roots were mechanically cleansed, and the samples were stored in 0.5% chloramine for 24 h.32
Next, a thin cotton-coated endodontic file was used to apply 2% formaldehyde on the apical foramen of each tooth. The apical foramens were sealed with self-curing acrylic and the roots were covered with die spacers up to the cementoenamel line in order to reduce dentinal permeability.
The samples were kept in deionized distilled water in a refrigerator at a nominal temperature of 4° C inside a container with an airtight seal that was periodically replaced in order to minimize deterioration until 24 hours prior to testing, when they were put in distilled water at 37° C, as recommended by the Colombian Technical Standard 4882.32
To test shear bond strength and to easily place the samples in the universal testing machine AG-SI Series Shimadzu (Shimadzu Corporation, Tokyo, Japan), cube-like holding molds were designed for embedding each tooth acrylic resin, exposing the enamel's buccal surface, after root amputation with a 2 mm diamond disc below the cementoenamel junction.
The exposed buccal enamel surface was abraded with #1000 SiC sandpaper to remove the prismless enamel layer, to improve the type of conditioning of the enamel surface, and to create a flat surface ensuring the application of pure shear force.32
The 90 teeth were randomly divided into six groups of fifteen teeth each, which were treated with an adhesive technique depending on the estimated time interval for each group as follows: control group, adhesion with no prior bleaching; group I, bleaching and immediate adhesion; group II, bleaching and adhesion one day later; group III, bleaching and adhesion seven days later; group IV bleaching and adhesion 14 days later; group V, bleaching and adhesion 28 days later. 38% hydrogen peroxide (Opalescence Xtra Boost®/Ultradent Products, South Jordan, USA) was applied according to the manufacturer's instructions, making three successive applications with a maximum thickness of 0.5 mm, letting each one act for a period of 8 min, without washing in between applications.
After completion of the whitening process, the bleaching agent was removed with running water, a very soft manual toothbrush,33,34 and non-fluoride toothpaste (FitoKids®, PF Farmacéutica SA/Tecser Laboratorios Ltda, Bogotá, Colombia).
The teeth were kept in artificial saliva (Salivary, Laboratorios Farpag, Bogotá, Colombia) at 37° C since the beginning of the whitening procedure until bond strength test according to the time interval of each group test; artificial saliva was replaced every two days.35
To begin the adhesive technique, the teeth were cleansed with baking soda and water. The area where the adhesive agent was applied was previously delineated by means of a Contact® paper circle of 6 mm in diameter with a 2 mm diameter hole in its center, in order to standardize the adhesion area.
The next step consisted on applying 35% phosphoric acid gel Scotchbond® (3M ESPE, St Paul, Minnesota, USA) as an etchant for 15 s; then the samples were washed and dried with absorbent paper, and two consecutive layers of the adhesive system Single Bond Plus® (3M ESPE, St. Paul, Minnesota, USA) were applied with a brush to facilitate impregnation until the surfaces turned bright; the samples were kept in position prior to aeration for 20 s. Next they were aired from 2 to 5 s and light cured with a halogen light Elipar® 2500 (3M ESPE, St Paul, Minnesota, USA) at an intensity of 600 mW/cm2 for 10 s and at a distance of 2 mm, following the manufacturer's instructions.36
The composite resin restorative material Filtek Z350 XT®, Color A2E (3M ESPE, St. Paul, Minnesota, USA) was introduced in a polypropylene tube of 2.08 mm in outer diameter and an inner light of 2 mm in diameter corresponding to the adhesive area. The aggregates were added in two increments of 1 mm each, using a stainless steel spatula with Teflon coating, and they were flattened on the prepared enamel surfaces in order to avoid bubbles and side excesses. Finally, the samples were light cured for 40 s at their mesial, distal, and vestibular surfaces.
After successful adhesion of the resin, the specimens were immersed in distilled water at 37° C for 24 h.
Later, the samples were brought to the universal testing machine AG-SI Shimadzu Series (Shimadzu Corporation, Tokyo, Japan), which contains a cutting blade that was placed at 0.5 mm from the bonding interface in order to apply a load of 50 N at a speed of 1 mm/min, and to record the values at which failure occurred. Since the adhesion area (A = ∏r2) was measured in mm2 and the load magnitude in Newtons, the final stress was calculated in MPa ((N/mm2).
The obtained data were processed with the TRAPEZIUM X operating software for material testing (Microsoft Windows®, USA) and later displayed on a table that correlates the two variables of the study: post-bleaching time intervals (0, 1, 7, 14, and 28 days) and bond strength to dentin in each test time (shear bond strength in MPa).
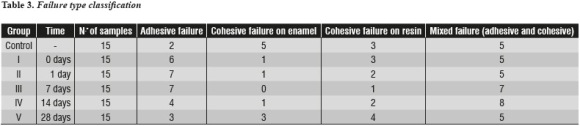
Once the specimens were fractured, the type of failure that occurred in each of the samples was determined by means of a Zoom® 2000 Leica stereomicroscope (Leica Microsystems, Buffalo NY, USA) at a 15X magnification.
Fractures were evaluated by classifying the type of failure, as follows: adhesive failure (> 75% failure between enamel and composite resin), cohesive failure in enamel (> 75% failure in enamel thickness), cohesive failure in resin (> 75% failure in composite resin thickness), and mixed failure (a combination of the above).
For shear bond strength testing, the data were analyzed by means of one-way analysis of variance (ANOVA) with the statistical F test and the Statistical Package for Social Sciences (SPSS) 15.0 (SPSS Inc., Chicago, IL, USA). Measures of central tendency (mean and standard deviation) were calculated with the use of Fisher and Duncan multiple comparisons—validated by homogeneity of variance with Levene's test—in order to summarize each group's data. P-values of < 0.05 were considered as statistically significant differences between the control group and the groups treated with bleaching at different time intervals.
RESULTS
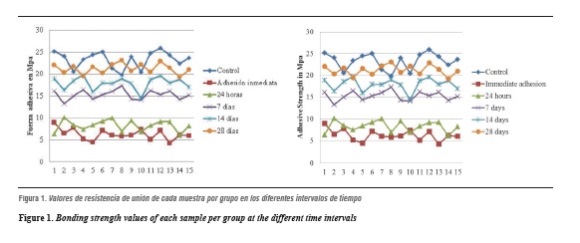
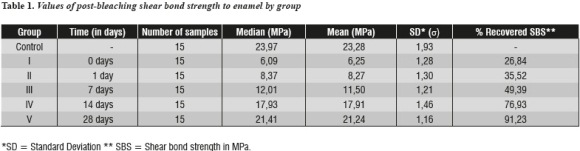
Figure 1 shows shear bond strength values for each sample per group at the time intervals analyzed. Mean and median values of shear bond strength obtained for each group are presented in table 1, which also shows the percentage of bonding strength recovery as post-bleaching time elapses.
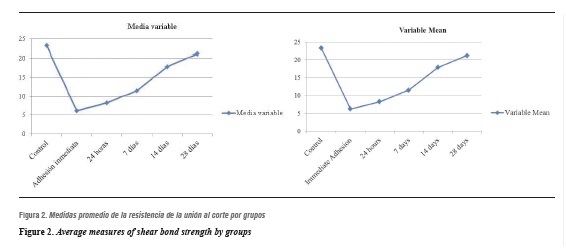
Figure 2 displays the means at each time interval. It shows that as time passes, shear bond strength is recovered. Note that on day 28 the average resistance approaches that of the control group.
Concerning variability within each group, variance behavior is homogeneous. This result means that the groups are comparable and that tests are needed to establish statistical differences between groups. Data analysis suggests that day 28 yields the best mean between the groups and that it is close to that of the control group.
With a confidence level of 95% so that the value of a (significance level) is 0.05, the first test shows no difference between the control group and the different exposure times, which suggests that at least one of the groups is statistically different from the control group (p < 0.001), offering statistical evidence to claim that at least one treatment is different.
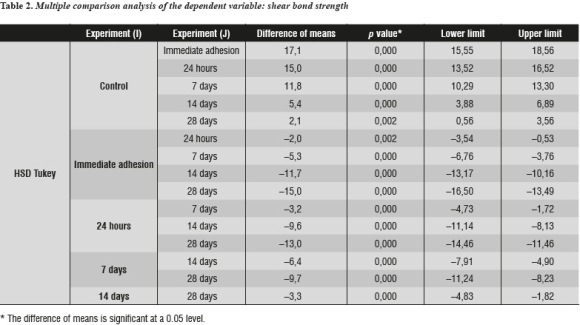
Table 2 shows bond strength values with differences within each group and their respective p values. The null hypothesis of equality between treatment means was rejected. In relation to the control group, a statistically significant decrease of postbleaching values of shear bond strength to dentin was observed in groups I (0 days p = 0.000), II (1 day p = 0.000), and III (7 days p = 0.000), IV (14 days p = 0.000) and V (28 days p = 0.002).
The Duncan test has a stronger composition, so the results confirm that there are statistical differences between all treatment means. The mean of group I is statistically lower than that of group II, this one is lower than that of group III, the one of group III is lower than the means of both group IV and group V, and finally this last group is statistically smaller than the control group.
The most common failure type was the mixed one (adhesive and cohesive). In some samples, the cohesive failure occurred on the enamel, while in others it happened on the resin or on the adhesive layer thickness. Furthermore, most samples from the control group experienced cohesive failures on the enamel; the majority of adhesive failures occurred between 0 and 14 days post-bleaching and during this time the frequency of cohesive failures on the resin increased. Cohesive failures on the resin decreased 28 days after bleaching, while the ones on the enamel increased during the same period—similarly to the behavior of the control group (table 3).
DISCUSSION
Studies such as the ones by Dishman et al,1 van der Vyver et al,2 Homewood et al,3 and Cappeletto et al4 have reported a transient decrease in composite resins' bond strength to enamel after bleaching.37, 38 Clinically, it is necessary to have relevant information on the behavior of bonding over time in order to perform adhesive cosmetic procedures such as replacing existing restorations, modifying shape and form, and cementing indirect veneers.37
With that in mind, the present study sought to evaluate the effect of 38% hydrogen peroxide on a composite resin shear bond strength, by performing the adhesive procedure at different time intervals (0, 1, 7, 14, and 28 days) after completion of teeth whitening treatment, in order to determine the period in which this procedure significantly affects adhesion to enamel and thus to be able to compare the results with those reported by other authors. In this study, we observed a significant reduction in adhesion after applying 38% hydrogen peroxide; we also found out that this effect is immediate and that it may last for days after bleaching, in agreement with the findings by Kimyai et al39 and Shinohara et al,24 who claim that applying bleaching agents in different concentrations adversely affects the immediate bonding of composite resins.
However, authors such as Dishman et al1 and Homewood et al3 concluded that after 24 hours there were no statistically significant differences in post-bleaching adhesion but they report a decrease in the formation of resin tags as well as inhibition of resin polymerization due to contact with residual oxygen. This theory may explain one of the possible reasons why in this study we observed a high frequency of adhesive and cohesive resin failures between 0 and 14 days, while the control group experienced cohesive enamel failures, suggesting that bonding strength at the adhesive interface is greater than that of the tissue's cohesive strength itself; such behavior is similar to that obtained 28 days after whitening, when cohesive resin failures decreased but enamel cohesive failures increased.
Van der Vyver et al2 reported a significant decrease in bond strength values, but only for the first two weeks, whereas under the conditions of the present study, adhesive bond strength alterations may take up to four weeks after completion of the bleaching treatment, and two weeks afterwards only a 76.93% of bond strength recovery was achieved—a result that may be explained by the findings of a previous study29 in which changes in the enamel chemical composition occurred following application of 38% hydrogen peroxide, producing a calcium reduction of 9.25%, an oxygen weight increase of 9.43% with respect to the control group, and after two weeks the percentage of oxygen was reduced by only 2.5%, indicating that this was not enough time for releasing all the residual oxygen from the enamel surface.
According to Suelieman et al,40 oxygen-free radicals and residual peroxide penetrate the enamel to reach dentinal tissue and even dental pulp, which hinders the release of bleaching agents' residual oxygen over time, apparently reducing adhesion due to alterations of the conversion degrees of both the adhesive system and the restorative composite resin.
Titley et al41 reported the formation of bubble-like porous zones of granular appearance in the adhesive interface due to retention of peroxide, which oxidizes on the enamel surface.
Miranda et al42 claim that the reduction in microtensile bond strength when using 22% carbamide peroxide may occur up to three weeks after application of the bleaching agent for two reasons: first, for a structural alteration associated with loss and erosion of the enamel prismless layer plus a reduction in the percentage of calcium and phosphorus; the second reason is of a residual nature, and is connected to the penetration of oxygen radicals in the enamel, which can inhibit polymerization.43
For the present study, it was necessary to remove the enamel's prismless layer in order to obtain a flat, standard and reproducible surface to properly apply cutting force during the bond strength test, following the recommendations of Colombian Technical Standard N° 4882.32 It has been reported that this layer is present in about 70% of permanent teeth44 and its thickness varies among the various types of teeth.45 Shi et al46 describe it as a surface layer highly mineralized compared with the enamel subsurface, and therefore more resistant to demineralization. Thus, the possible protective function of this structure is lost, and as a consequence the bleaching agent's structural and chemical effects occur at the enamel's prism area affecting the surface to be conditioned and therefore reducing bond strength.28
Josey et al28 suggest that post-bleaching etching produces a structural loss at the limits of the enamel prisms, and that such changes may affect the adhesive systems' retention qualities due to inadequate formation of resin tags. Accordingly, bond strength decrease on previously whitened teeth may generally be attributed to two possible factors: microstructural modification of the surface and distribution of residual hydrogen peroxide and oxygen-free radicals over the tooth structure.
Differences in the data reported by several authors, including the present study, concerning the time required for composite resins to recover bond strength may be due to several factors, including the type of bleaching agent used (carbamide peroxide, hydrogen peroxide, sodium perborate, or ozone), as they all differ in the amount of peroxide that has the ability to degrade to oxygen. 10% carbamide peroxide is decomposed to 3% hydrogen peroxide and urea, so the amount of released gaseous oxygen molecules is also reduced, unlike peroxides of high concentration, which are considered to increase the likelihood of the enamel's surface and subsurface suffering structural damage (see Hegedüs et al).47
Furthermore, Pérez et al48 argue that morphological changes of the enamel surface are related to exposure time and bleaching agent concentration, and that an exposure time longer than 20 hours produces histological changes in enamel prisms—an effect that can interfere with the enamel etching pattern—.21 Some other studies report changes in bleaching agents behavior with respect to tooth structures due to addition of other substances such as carbopol,49 fluorine,50, 51 or amorphous calcium phosphate.52, 53 If these substances are included in the product or if they are used before or after the whitening procedure, they may produce microstructural changes or alter the enamel's mechanical properties, such as surface hardness.50, 54
It is important to point out that our samples were kept in artificial saliva until the time of testing in order to better simulate intra-oral conditions, but as suggested by authors such as Cappeletto et al,4 despite prolonged in vitro exposure to artificial saliva with the intention of reversing the deleterious effects of residual oxygen, rapid neutralization of hydrogen peroxide cannot be achieved. In vivo, it may be possible to achieve adequate levels of adherence to enamel in less time than the required in this study thanks to the remineralizing and antioxidant action of salivary components.
Different techniques have been suggested to reduce waiting times for post-bleaching bonding procedures, such as removal of the enamel's outer surface layer,55 the use of enzymatic antioxidants such as catalase56 and peroxidase,57 or non-enzymatic antioxidants such as sodium ascorbate,57.58 and the application of ethanol,59 sodium bicarbonate, and vitamin E,60 as alternatives for removing residual molecules from the enamel surface and thus recovering the bonding strength of composite resins adhered to enamel and preventing adverse biological effects.61
In the search for components able to reduce the problems caused by bleaching agents on enamel, some studies have been conducted on the use of fluoride for stabilizing the cyclic process of ion exchange to which teeth are subjected. Fluorine atoms replace the hydroxyl ion (OH) to form fluorapatite—a compound that has shown greater resistance to pH changes and therefore to demineralization—. Türkün et al59 and Da Costa et al62 reported that topical application of fluoride has the ability to reverse the adverse effects of demineralization caused by the application of bleaching agents, such as reduction of the enamel surface hardness.
We used fluoride-free toothpaste in our study with the intention of applying a substance that would completely remove peroxide by a mechanical effect of brushing, and to avoid modifying the structure by applying remineralizing substances.
Additionally, the toothpaste we used contains sodium lauryl sulfate, a cleansing substance that works as a detergent and has the ability to penetrate and eliminate surface impurities. However, Barkvoll63 and other authors have pointed out that this substance can reduce the cariostatic effect of fluoride when in combination with sodium monofluorophosphate during topical application and that it may participate in the deposition of fluoride on enamel. This was not the case of the composition of the toothpaste used in this study, but it is important to note that even though there are no specific reports on the possible influence of lauryl sulfate on the deposition of calcium and phosphate ions on the enamel surface layer, another interaction may occur in a similar way to the one that favored fluorapatite formation, inhibiting the protective remineralizing action pursued by using artificial saliva as a preserving agent during the pilot phase and as a way to imitate the normal conditions of the oral cavity in vivo.
Moreover, FitoKids® (PF Farmacéutica SA/Tecser Laboratorios Ltda, Bogotá, Colombia) contains extracts of Calendula officinalis, Salvia officinalis and Cardamom, three plants that are considered to have antioxidant, estrogenic and anti-inflammatory properties.64-67 The components of this toothpaste are based on active ingredients known for their biological activity such as alkaloids, saponins, flavonoids, tannins, terpenes, or coumarins.68
This wide spectrum of chemical components is consistent with the diversity of pharmacological actions of these plants. Their antioxidant function results from their content of polyphenols—low-molecular weight phytochemicals that form not only simple molecules such as phenolic acid and flavonoids but also highly structured compounds such as tannins—. Their antioxidant activity stems from their high reactivity as electron donors and from the ability of formed radicals to stabilize unpaired electrons and to stop chain reactions. Flavonoids have a powerful antioxidant action in vitro, being able to act on a wide range of reactive oxygen, nitrogen, and chlorine species, such as superoxide, the hydroxyl radical, the perhydroxyl radical, and hypochlorous acid, which function as reducing agents.69, 70
It is important to consider that, according to the results achieved in terms of bond strength following post-bleaching surface cleaning, apparently there was not a positive influence of the substance as an antioxidant agent, which may be due to the relationship between the concentrations of both peroxide and antioxidant, as supported by Freire et al,71 who reported that antioxidants are useful in removing oxygen-free radicals from surfaces, providing that there is a direct relationship between the concentration of hydrogen peroxide and the concentration of antioxidant. They also claim that in order to completely remove 2 g of 35% hydrogen peroxide, 20 ml of sodium ascorbate are required at a concentration of at least 25%. A solution with lower concentrations is therefore considered to be ineffective to neutralize the radicals produced by 38% hydrogen peroxide.
Regarding time of application of the product as a possible antioxidant, Lai et al72 maintain that the required time to leave antioxidants act on the tooth surface is at least a third of the time spent on the tooth whitening procedure so, for example, if tooth whitening lasted for 8 hours, the antioxidant must be in contact with the surface for at least 3 hours.
This parameter was not met in the present study, as the bleaching agent was applied according to the manufacturer's instructions for 24 minutes (3 applications of 8 minutes each) and the product was in contact with the tooth surface for about 30 seconds. This time was not effective to achieve recovery of adhesive values from 0 to 28 days post-bleaching, showing little influence of the cleaning substance.
As bond strength decrease has been attributed to the alteration of crystal structures' calcium-phosphate ratio,9, 73 as well as to microstructural changes of hydroxyapatite crystals, and therefore to inadequate etching patterns on the enamel during the adhesive technique,74, 75 various products have been used to achieve a remineralization effect on the tooth structure that has been altered—a mechanism defined by Cochrane NJ 76 as the process by which ions are supplied from a source outside the tooth to promote ion deposition on the crystals in a demineralized enamel in order to produce mineral network gain, and thereby to ensure a better tissue quality in presence of an adhesive technique.
To achieve this effect, several substances have been tested, including sodium fluoride74,77, and calcium, 78 which acts as a mineralizing agent that inhibits mineral loss and prevents surface microhardness decrease, as well as the amorphous calcium phosphate in its crystalline form, which has been proven to be difficult to achieve adequate enamel remineralization due to low solubility of calcium phosphate phases, so that calcium and phosphate ions are not available for remineralization.76 Similarly, Da Costa62 and Tschoppe et al79 have reported that both potassium nitrate and amorphous calcium phosphate lack the ability to prevent enamel surface hardness decrease.
Tricalcium phosphate (TCP) has also been tried in dental products, as it has been proven to be effective to improve the microhardness of demineralized enamel surfaces compared to products with fluoride alone, possibly due to an abrasive effect. However, there are no published studies on this material as to demonstrate its ability to remineralize enamel subsurface lesions.76
With respect to amorphous calcium phosphate stabilized by casein phosphopeptides (CPP -ACP), an increase in microhardness after bleaching has been found in samples bleached with hydrogen peroxide using CPP-ACP paste before and after bleaching, suggesting a mineral deposition on the enamel. The CPP-ACP system provides a superior deposit of bioavailable calcium as well as phosphate ions in comparison with ACP alone, leading to higher potential remineralization.76
Since there is still much controversy on the adverse effects of bleaching agents on tooth structure and post-bleaching adherence, there is also a clear need of reconsidering clinical protocols to ensure a better management of bleaching agents and remineralizing substances as well as the time required to achieve neutralization and remineralization of tooth structure post-bleaching.
CONCLUSIONS
- Given the conditions under which this study was developed, the following conclusions may be drawn:
- A significant reduction of a composite resin`s shear bond strength to dentin occurs after the application of 38% hydrogen peroxide until 28 days post-bleaching.
- The bond strength obtained at different time intervals is statistically lower than that of the control group. Group V (28 days) descriptively resembles the control group but is still statistically lower, so the waiting time should be greater than 28 days if we want to recover 100% of the shear bond strength values of the resin adhering to dental enamel post-bleaching.
RECOMMENDATIONS
It is necessary to perform new studies involving easy-to-use and inexpensive antioxidants in order to decrease the time needed to neutralize residual oxygen post-bleaching and to achieve dental restorations bonded to enamel without waiting too long.
CONFLICT OF INTEREST
The authors declare having no conflict of interest in the present study.
REFERENCES
1. Dishman MV, Covey DA, Baughan LW. The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater 1994; 10(1): 33-36. [ Links ]
2. Van der Vyver PJ, Lewis SB, Marais JT. The effect of bleaching agent on composite/enamel bonding. J Dent Assoc S Afr 1997; 52(10): 601-603. [ Links ]
3. Homewood C, Tyas M, Woods. Bonding to previously bleached teeth. Aust Orthod J 2001; 17(1): 27-34. [ Links ]
4. Cappeletto E, Pedroso C, Takeo A, Campos M. Influence of post-bleaching time intervals on dentin bond strength. Braz Oral Res 2004; 18(1): 75-79. [ Links ]
5. Haywood VB, Leonard RH, Nelson CF, Brunson WD. Effectiveness, side effects and long-term status of nightguard vital bleaching. J Am Dent Assoc 1994; 125(9): 1219-1226. [ Links ]
6. Dahl J, Pallesen U. Tooth bleaching a critical review of the biological aspects. Crit Rev Oral Biol Med 2003; 14(4): 292-304. [ Links ]
7. Cobankara FK, Unlun N, Altinoz HC, Fusun O. Effect of home bleaching agents on the roughness and surface morphology of human enamel and dentine. Int Dent J 2004; 54(4): 211-218. [ Links ]
8. Pinto CF, Oliveira R, Cavalli V, Giannini M. Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology. Braz Oral Res 2004; 18(4): 306-311. [ Links ]
9. Gotz H, Duschner H, White DJ, Klukowska A, Malgorzata A. Effects of elevated hydrogen peroxide 'strip' bleaching on surface and subsurface enamel including subsurface histomorphology, micro-chemical composition and fluorescence changes. J Dent 2007; 35: 457-466. [ Links ]
10. Claus PE. Effects of hydrogen peroxide-containing bleaching agents on the morphology of human enamel. Quintessence Int 1996; 27(1): 53-56. [ Links ]
11. Moreira de Freitas PM, Turssi CP, Hara AT, Serra MC. Dentin microhardness during and after whitening treatments. Quintessence Int 2004; 35(5): 411-417. [ Links ]
12. Romero AD, Bueno GJ. Radicales libres del oxígeno y antioxidantes en medicina. Rev Clin Española 1998; 184(7): 345-346. [ Links ]
13. Joiner E. The bleaching of teeth: A review of the literature. J Dent 2006; 34(7): 412-419. [ Links ]
14. Goldstein RE, Garber DA. Complete dental bleaching. Chicago: Quintessence Publishing; 1995. [ Links ]
15. Domínguez MN, González LS, Menéndez NM. Study of the diffusion ways in the white spot enamel lesion. RCOE 2002; 7(5): 469-476. [ Links ]
16. Price RBT, Sedarous M, Hiltz GS. The pH of tooth-whitening products. J Can Dent Assoc 2000; 66: 421-426. [ Links ]
17. Cheesman KH, Slater TF. Free radicals in medicine. Br Med Bull 1998; 49: 118-121. [ Links ]
18. Floyd RA. The effect of peroxides and free radicals on body tissues. J Am Dent Assoc 1997; 128: 37-44. [ Links ]
19. Venereo JR. Daño oxidativo, radicales libres y antioxidantes. Rev Cub Med Mil 2002; 31(2): 126-133. [ Links ]
20. Mc Guckin RS, Thurmond BA, Osovitz S. Enamel shear bond strengths after vital bleaching. Am J Dent 1992; 5: 216-222. [ Links ]
21. Titley KC, Torneck CD, Smith DC, Chernecky R, Adibfar A. Scanning electron microscopy observation on the penetration and structure of resin ''tags'' in bleached and unbleached bovine enamel. J Endod 1991; 17: 72-75. [ Links ]
22. Carvalli V, Giannini M, Carvalho R. Effect of carbamide peroxide bleaching agents on tensile strength of human enamel. Dent Mater 2004; 20: 733-739. [ Links ]
23. Rotstein I. Role of catalase in the elimination of residual hydrogen peroxide following tooth bleaching. J Endod 1993; 19: 567-569. [ Links ]
24. Shinohara MS, Peris AR, Pimenta LA, Ambrosano GM. Shear bond strength evaluation of composite resin on enamel and dentin after nonvital bleaching. J Esthet Restor Dent 2005; 17(1): 22-29. [ Links ]
25. Sung EC, Chan SM, Mito R, Caputo AA. Effect of carbamide peroxide bleaching on the shear bond strength of composite to dental bonding agent enhanced enamel. J Prosthet Dent 1999; 82(5): 595-599. [ Links ]
26. Cadenaro M, Breschi L, Antoniolli F, Mazzoni A, Di Lenarda R. Influence of whitening on the degree of conversion of dental adhesives on dentin. Eur J Oral Sci. 2006; 114(3): 257-262. [ Links ]
27. Ben-Amar A, Liberman R, Gorfil C, Bernstein Y. Effect of night guard bleaching on enamel surface. Am J Dent 1995; 8: 29-32. [ Links ]
28. Josey AL, Meyers IA, Romaniuk K, Symons AL. The effect of vital bleaching technique on enamel surface morphology and the bonding of composite resin to enamel. J Oral Rehabil 1996; 23: 244-250. [ Links ]
29. Baldión PA, Arcos LC, Mora MA. Efecto de los fluoruros en la composición química del esmalte dental posblanqueamiento. Univ Odontol 2011; 30(65): 41-49. [ Links ]
30. Manzini JL. Declaración de Helsinki: Principios éticos para la investigación médica sobre sujetos humanos. Acta Bioethica 2000; 6(2): 321-334. [ Links ]
31. Colombia Ministerio de Salud. Resolución N.° 8430 de 1993, octubre 4, por la cual se establecen las normas científicas, técnicas y administrativas para la investigación en salud. Título II. De la investigación en seres humanos. Capítulo 1. De los aspectos éticos de la investigación en seres humanos: Artículos 4 al 16. Bogotá: El Ministerio; 1993. [ Links ]
32. Comité Materiales Odontológicos. NTC 4882. Métodos de ensayo para la evaluación de la unión adhesiva entre los materiales odontológicos y la estructura dental. Instituto Colombiano de Normas Técnicas y Certificación, ICONTEC. Sector: 11-Tecnología del cuidado de la salud. Fecha de ratificación: 25/10/2000. Actualización: Ninguna. [ Links ]
33. Tschoppe P, Zandim DL, Martus P, Kielbassa AM. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J Den 2011; 39(6): 430-437. [ Links ]
34. Tschoppe P, Meyer-Lueckel H. Effects of regular and highly fluoridated toothpastes in combination with saliva substitutes on artificial enamel caries lesions differing in mineral content. Arch Oral Biol 2012; 57(7): 931-939. [ Links ]
35. Tschoppe P, Kielbassa AM, Meyer-Lueckel H. Evaluation of the remineralising capacities of modified saliva substitutes in vitro. Arch Oral Biol 2009; 54(9): 810-816. [ Links ]
36. 3M ESPE Casa comercial. Perfil técnico del producto: Adpter® Single Bond. Sistema adhesivo dental. [en línea] [fecha de acceso 15 de octubre de 2012 ]; URL disponible en: http://multimedia.3m.com/mws/mediawebserver? UUUUUUC04ehUnx7UGx7UUUPJtEtttttS- [ Links ]
37. Torneck CD, Titley KC, Smith DO, Adibfar A. Effect of water leaching on the adhesion of composite resin to bleached and unbleached enamel. J Endodon 1991; 17: 156-160. [ Links ]
38. Gokce B, Comlekoglu ME, Ozpinar B, Turkun M, Demirbas AK. Effect of antioxidant treatment on bond strength of a luting resin to bleached enamel. J Dent 2008; 36: 780- 785. [ Links ]
39. Kimyai S, Oskoee SS, Rafighi A, Valizadeh H, Ajami AA, Helali ZZ. Comparison of the effect of hydrogel and solution forms of sodium ascorbate on orthodontic bracket- enamel shear bond strength immediately after bleaching: An in vitro study. Indian J Dent Res 2010; 21: 54-58. [ Links ]
40. Suelieman M, Addy M, Macdonald E, Ress J. The leaching depth of a 35% hydrogen peroxide based in-office product: a study in vitro. J Dent 2005; 33: 33-40. [ Links ]
41. Titley KC, Torneck CD, Ruse ND. The effect of carbamide- peroxide gel on the shear bond strength of a microfilm resin to bovine enamel. J Dent Res 1992; 71: 20-24. [ Links ]
42. Miranda AM, Bermejo GN, Bazan JE, Saravia MA. Efectos de un blanqueamiento dental con ozono y otro con peróxido de carbamida al 22% sobre la fuerza de adhesión al esmalte en diferentes intervalos de tiempo. Acta Odontol Venez 2009; 47(4): 69-77. [ Links ]
43. Cavalli V, Reis AF, Giannini M, Ambrosano GM. The effect of elapsed time following bleaching on enamel bond strength of resin composite. Oper Dent 2001; 26: 597-602. [ Links ]
44. Gómez ME, Campos A. Esmalte. En: Histología y embriología bucodental. 2ed. Madrid: Médica Panamericana; 2003. p. 273-316. [ Links ]
45. Whittaker DK. Structural variations in the surface zone of human tooth enamel observed by scanning electron microscopy. Archs Oral Biol 1982; 27(5): 383-392. [ Links ]
46. Shi XC, Ma H, Zhou JL, Li W. The effect of cold-light-activated bleaching treatment on enamel surfaces in vitro. Int J Oral Sci 2012; 4: 208-213. [ Links ]
47. Hegedüs C, Bistey T, Flóra-Nagy E, Keszthelyi G, Jenei A. An atomic force microscopy study on the effect of bleaching agents on enamel surface. J Dent 1999; 27: 509-515. [ Links ]
48. Pérez LF, Díaz AM, Aguirre M, Alcántara CM, Aguilar RE, Acedo JE et al. Efecto del peróxido de carbamida sobre el esmalte dentario a diferentes concentraciones y tiempos de exposición (estudio in vitro). Odontol Sanmarquina 2004; 8(1): 25-29. [ Links ]
49. Rodrígues JA, Oliveira GP, Amaral CM. Effect of thickener agents on dental enamel microhardness submitted to at-home bleaching. Braz Oral Res 2007; 21(2): 170-175. [ Links ]
50. Chen HP, Chang CH, Liu JK, Chuang SF, Yang JY. Effect of fluoride containing bleaching agents on enamel surface properties. J Dent 2008; 36(9): 718-725. [ Links ]
51. Dominguez JA, Bittencourt B, Michel M, Sabino N, Gomes JC, Gomes OM. Ultrastructural evaluation of enamel after dental bleaching associated with fluoride. Microsc Res Tech 2012; 75(8): 1093-1098. [ Links ]
52. Adebayo OA, Burrow MF, Tyas MJ. Effects of conditioners on microshear bond strength to enamel after carbamide peroxide bleaching and/or casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) treatment. J Dent 2007; 35(11): 862-870. [ Links ]
53. Zhao J, Liu Y, Sun WB, Zhang H. Amorphous calcium phosphate and its application in dentistry. Chem Cent J 2011; 5: 40. [ Links ]
54. Goswami M, Saha S, Chaitra TR. Latest developments in non-fluoridated remineralizing technologies. J Indian Soc Pedod Prev Dent 2012, 30(1): 2-6. [ Links ]
55. Cvitko E, Deheny GE, Swift Jr EJ, Pires JA. Bond strength of composite resin to enamel bleached with carbamide peroxide. J Esthet Dent 1991; 3: 100-102. [ Links ]
56. Rotstein I, Dankner E, Goldman A, Heling I, Stabholz A, ZalkindM. Histochemical analysis of dental hard tissues following bleaching. J Endod 1996; 22: 23-26. [ Links ]
57. Baldión PA, Viteri LN, Lozano E. Efecto de la peroxidasa sobre la resistencia de unión de una resina compuesta al esmalte dental posblanqueamiento. Rev Fac Odontol Univ Antioq 2012; 24(1): 8-21. [ Links ]
58. Kaya AD, Türkün M. Reversal of dentin bonding to bleached teeth. Oper Dent 2003; 28(6): 825-829. [ Links ]
59. Türkün M, Kaya AD. Effect of 10% sodium ascorbate on the shear bond strength of composite resin to bleached bovine enamel. J Oral Rehab 2004; 31: 1184-1191. [ Links ]
60. Barghi N, Godwin JM. Reducing the adverse effect of bleaching on composite–enamel bond. J Esthet Dent 1994; 6: 157-161. [ Links ]
61. García EJ, Oldoni TL, Alencar SM, Reis A, Loguercio AD, Grande RH. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz Dent J 2012; 23: 22-27. [ Links ]
62. Da Costa DB, Mazur RF. Effects of the new formulas of bleaching gel and fluoride application on enamel microhardness: An in vitro study. Oper Dent 2007; 32: 589-594. [ Links ]
63. Barkvoll P. Effect of sodium lauryl sulfate on the uptake of fluoride from NaF and MFP by etched enamel in vitro. J Biol Buccale 1991; 19(3): 235-239. [ Links ]
64. Kennedy DO, Scholey AB. The psychopharmacology of European herbs with cognition-enhancing properties. Curr Pharm Des 2006; 12(35): 4613-4623. [ Links ]
65. Dedio I. Value of Calendula officinalis as a tannin source. Herba Pol 1983; 29(3-4): 211-216. [ Links ]
66. Brasseur T, Abgenot L, Pricemail J, Debev C. Free radical formation inhibiting and antioxidant properties of flavonoids. J Exptl Biol Med 1986; 37: 533-548. [ Links ]
67. Groppo FC, de Cássia BC, Cogo K, Franz-Montan M, Lopes RH, Dias AE. Use of phytotherapy in dentistry. Phytother Res 2008; 22(8): 993-1133. [ Links ]
68. Lu Y, Foo LY. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry 2000; 55(3): 263-267. [ Links ]
69. Halliwell B. Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? Am J Clin Nutr 2005. 81(suppl): 268-276. [ Links ]
70. Roginsky V. Chain-breaking antioxidant activity of natural polyphenols as determined during the chain oxidation of methyl linoleate in Triton X-100 micelles. Arch Biochem Biophys 2003; 414: 261-270. [ Links ]
71. Freire A, Souza E, Biazzetto D, Ribeiro E, Cynthia C, Marins R et al. Reaction kinetics of sodium ascorbate and dental bleaching gel. J Dent 2009; 37: 932-936. [ Links ]
72. Lai S, Mak Y, Cheung G, Osorio R, Toledano M. Reversal of compromised bonding to oxidized etched dentin. J Dent Res 2001; 80: 1919-1924. [ Links ]
73. Tezel H, Ertas OS, Ozata F, Dalgar H, Korkut ZO. Effect of bleaching agents on calcium loss from the enamel surface. Quintessence Int 2007; 38(4): 339-347. [ Links ]
74. Attin T, Kielbassa AM, Schwanenberg M, Hellwig E. Effect of fluoride treatment on remineralization of bleached enamel. J Oral Rehabil 1997; 24: 282-286. [ Links ]
75. Attin T, Hanning G, Wiegand A, Attin R. Effect of bleaching on restorative materials and restorations. A systematic review. Dent Mater 2004; 20: 852-861. [ Links ]
76. Cochrane NJ, Cai F, Huq NL, Burrow MF, Reynolds EC. New approaches to enhanced remineralization of tooth enamel. J Dent Res 2010; 89: 1187-1197. [ Links ]
77. Lewinstein I. Effect of different peroxide bleaching regimens and subsequent fluoridation on the hardness of human enamel and dentin. J Prosthet Dent 2004; 92: 337-342. [ Links ]
78. Cavalli V, Rodrigues LK, Paes-Leme AF, Brancalion ML, Arruda MA, Berger SB, et al. Effects of bleaching agents containing fluoride and calcium on human enamel. Quintessence Int 2010; 41: 157-165. [ Links ]
79. Tschoppe P, Neumann K, Mueller J, Kielbassa AM. Effect of fluoridate bleaching gels on the remineralization of predemineralized bovine enamel in vitro. J Dent 2009: 37(2): 156-162. [ Links ]











 text in
text in