Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Odontología Universidad de Antioquia
Print version ISSN 0121-246X
Rev Fac Odontol Univ Antioq vol.26 no.1 Medellín July/Dec. 2014
ORIGINAL ARTICLES DERIVED FROM RESEARCH
IN VITRO EVALUATION OF THE EFFECT OF HYDROFLUORIC ACID ONCENTRATION AND APPLICATION TIME ON ADHESION TO LITHIUM DISILICATE
Carlos Caparroso Pérez1; Federico Latorre Correa2; Luz Janeth Arroyave Hoyos3; Carlos Andrés Grajales Gaviria;3 Verónica María Medina Piedrahita3
1 Dentist, Specialist in Comprehensive Dentistry of Adults, Magister in
Higher Education, Associate Professor, School of Dentistry, Universi-
dad de Antioquia. Email address: ccaparroso@gmail.com
2 Dentist, Specialist in Comprehensive Dentistry of Adults, Magister in
Higher Education, Associate Professor, School of Dentistry, Universidad
de Antioquia. Email address: latorre.federico29@gmail.com
3 Graduate students, Comprehensive Dentistry of Adults with a focus on
Prosthodontics. School of Dentistry, Universidad de Antioquia
SUBMITTED: FEBRUARY 8/2013-ACCEPTED: NOVEMBER 19/2014
Caparroso CB, Latorre F, Arroyave LJ, Grajales CA. In vitro evaluation of the effect of hydrofluoric acid concentration and application time on adhesion to lithium disilicate. Rev Fac Odontol Univ Antioq 2014; 26(1): 62-75.
ABSTRACT
INTRODUCTION: as a ceramic restorative material, lithium disilicate provides higher resistance to ceramic materials, excellent
optical characteristics, and the ability to be bonded to tooth substrate by hydrofluoric acid etching. The purpose of this study was to perform in
vitro assessment of the effect of 4.6 and 9.5% hydrofluoric acid at 20, 40 and 60 s on the surface of lithium disilicate during the processes of
adherence to a resinous substrate.
METHODS: 6 IPS e-max® Press porcelain cubes and 6 Filtek Z250®
resin cubes were made. The ceramic cubes
were subjected to surface treatment obtaining 6 groups: control group and groups 2 and 3 were treated with 4.6% hydrofluoric acid during 20,
40 and 60 seconds respectively, and groups 4, 5 and 6 were treated with 9.5% hydrofluoric acid at 20, 40 and 60 s respectively. Porcelain cubes
were cemented with the resin ones with self-adhesive resin cement and bars of 1 x 1 x 18 mm were produced, obtaining 20 samples in each group.
Adhesive strength was measured.
RESULTS: adhesive strength values expressed in MPa (SD) were as follows: G1 = 26.69 (± 7.99), G2 = 23.93
(± 7.99), G3 = 20.90 (± 7.91), G4 = 17.56 (± 6.61), G5 = 16.30 (± 11.68), G6 = 18.78 (± 10.22), with significant differences between group 1
and groups 4-5.
CONCLUSIONS: 4.6% hydrofluoric acid produced better adhesive averages compared with the 9.6% concentrations. The group
with 4.6% concentration during 20 s offered the best results.
Key words: ceramics, dental porcelain, lithium disilicate, hydrofluoric acid.
INTRODUCTION
Feldspathic ceramics have a mixed structure composed of a crystalline phase and a glassy phase.1 This material produces retention in a micromechanical way, by means of a sandblasted surface or by acid and chemical etching with bonding agents or silanes.2 The crystalline phase offers better mechanical characteristics, such as the ceramics with a high content of alumina and zirconia, but as a disadvantage they decrease values of adhesion and translucency.2 Lithium disilicate-based ceramics have 60-70% of crystals embedded in a vitreous matrix, which provide them with increased resistance compared to feldspathic ceramics; also, they can be prepared by acid etching.3
Resinous compounds' adhesion to ceramics with feldspar, leucite, and lithium disilicate has been well established. Hydrofluoric acid attacks the glassy phase and forms a retentive surface for micromechanical adhesion, and silane, as a bonding agent, promotes chemical bonding between these ceramics' silica and methacrylate resin groups.2 The process of adhesion to silica for cementation of lithium disilicate ceramics is made by surface etching with hydrofluoric acid.4 The most commonly used concentrations are 4.6 and 9.5%. IPS e.max Press ceramics was first introduced in the profession in 2005, and consists of 70% of lithium disilicate crystals (Li2Si2O5), which are embedded in a vitreous matrix.5 For this material, the manufacturer recommends using 4.6% hydrofluoric acid for 20 seconds.6
Some studies have analyzed the effect of surface treatment with hydrofluoric acid on lithium disilicate adhesion. Kiyan et al7 found out that preparation of a sandblasted surface treated with 10% hydrofluoric acid produces adhesion values of about 13.65 MPa. Spohr et al8 found out that etching under the same conditions with and without application of a bonding agent or silane produces adhesion values of 25.6 and 16.4 MPa respectively. By using the same surface treatment, Meyer et al9 found adhesion values of 35.1 and 56.8 MPa respectively. By increasing etching time to 30 s with the same concentration, Gökçe et al10 obtained higher levels of adherence to the ceramic substrate. Pollington et al11 found that increasing etching time to 60 s with the same concentration of etching agent produces an adhesion strength of 24.76 MPa. Other authors, such as Kim et al,12 using a concentration of 4% hydrofluoric acid for 5 min produced levels of adhesion of 17.7 MPa. These studies did not make comparisons between application times and hydrofluoric acid concentration in their cementation protocols. The purpose of this study was to evaluate the effect of 4.6 and 9.5% hydrofluoric acid at different application times (20, 40 and 60 s) on lithium disilicate surface during the processes of adherence to a resinous substrate. The results pretend to provide a scientific basis to the process of etching the surface of lithium disilicate restorations with hydrofluoric acid.
METHODS
Six 10 x 10 mm (± 0,2 mm) porcelain cubes were made in IPS e-max® Press ceramics through the lost-wax technique following the manufacturer's instructions (Ivoclar VivadentTM).13 The samples were coated on Emax Press Speed refractory material, in a proportion of 32 ml of special liquid by 22 ml of distilled water and 200 g of powder, vacuum mixing with Bego Easy Mix equipment during 90 seconds. After forging for 45 min, the rings were taken to the oven to a temperature of 850° C for 60 min. The injection was done in a Programat EP5000 oven to a temperature of 915° C. Each sample was sandblasted with aluminum oxide particles at a pressure of two bars (30 psi) for about 14 s at a distance of 10 mm.9
Six Filtek® Z250 resin cubes were made with the same dimensions, using an incremental technique with polymerization periods of 20 s. Two fixing tabs were placed on each cube to secure them at the time of sectioning. Finally, all the samples were vaporized with a Zhermack VAP 6A machine in order to remove any remnants (table 1 and figure 1).13
Each ceramic cube was subjected to surface treatment with different hydrofluoric acid concentrations and exposure times, creating six groups to be assessed: In Group I (control group) we used IPS Ceramic Etching Gel 4.6% hydrofluoric acid during 20 s (IPS-20). In groups II and III we used the same concentration of acid, with application times of 40 s (IPS-40) and 60 s (IPS-60). In groups IV, V, and VI we used Eufar® 9.6% hydrofluoric acid for 20 s (Eufar-20), 40 s (Eufar-40) and 60 s (Eufar-60), respectively (table 2).
Following the acid etching protocol, each sample was washed with abundant water for 30 s, and applied Silano Monobond-S®, Ivoclar Vivadent® using a disposable brush; they were left to air dry according to the manufacturer's instructions. The porcelain cubes were cemented to the resin cubes by means of a self-adhesive resinous cement (Relyx U100®, 3M-Espe), which is routinely used in this type of restorations.14-16 This material was prepared according to the manufacturer's instructions and brought to the adhesive surface with a plastic spatula.17 Samples were kept in position by means of digital pressure and polymerized with a source of light of 1100 to 1250 mW/cm2 in intensity. The process of handling and curing lasted for 40 seconds every 90° at the lithium disilicate-resin interface. We also performed intra- and inter-operator standardization for the processes of sample formation and cementation. Each sample was stored during 1 hour outdoors and 23 hours in distilled water at 37° C. Thermal cycling of samples was performed at 5,000 cycles, with temperature changes ranging from 5 to 55° C during 30 s and a transfer time of approximately 2 s.
The resulting cubes were sectioned perpendicularly to the cementing agent, with a 0.22 mm thick diamond disc, using a Buehler Isomet 1000 Precision cutting machine, at a cutting speed of 250 RPM and a counterweight of 200 g (figure 2), thus generating bars of 1 x 1 x 18 mm (± 0.3 mm). Each bar was measured with a Mutitoyo digital gauge in order to standardize the sample and to determine the strength per unit of area required for failure during the adhesion test. Adhesion values were obtained by means of a micro tensile strength test, with 1 x 1 x 18 mm bars, in order to reduce the probability of defects at the adhesive interface and therefore a reduction in its strength. It has been shown that the adhesion interface modifies tensile resistance; the smaller the area, the greater the test reliability.18-21
Once the sectioning phase was completed, 20 samples from each group were randomly selected for a total of 120 samples to be evaluated (figure 3).
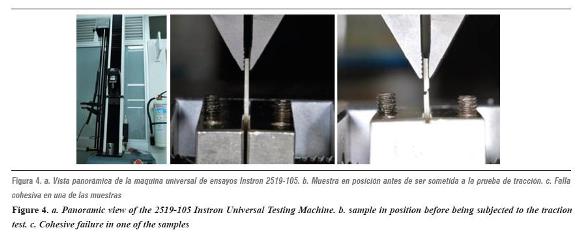
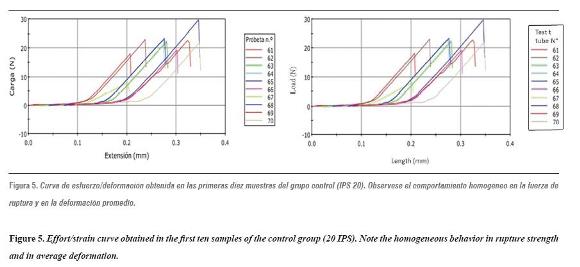
The universal testing machine was calibrated using 20 additional samples as a pilot test. The samples were subjected to a load in a 2519-105 Instrontm Universal Testing Machine, with a capacity of 50 Newtons, in order to test adhesive strength. A charge speed of 10 mm/min was used and adhesion to lithium disilicate was recorded until producing some kind of failure on the effort/strain curve (figures 4 and 5).
Rupture strength was measured in Newtons, then divided by a transversal section of each sample's area, and finally expressed in Mega Pascals (MPa),11 so that adhesion in MPa = P/A, where P is the maximum force (N) and A is the area of each sample (mm2 ).5
Statistical data analysis was made with version 19 of SPSS. Data were expressed in averages with their respective standard deviation (variables with normal distribution according to the Kolmogorov- Smirnov test). Comparisons were made by one- way ANOVA variance analysis, and it was decided that, if this test was ever applied obtaining P > 0.05, a Post Hoc analysis would be conducted.
RESULTS
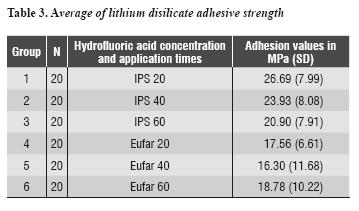
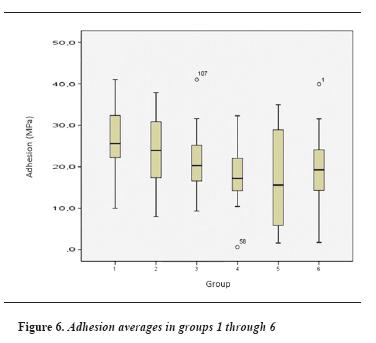
Each group's adhesion values were obtained by micro tensile strength test until failure; these values are shown in table 3 and figure 6.
Adhesive strength averages and their standard deviation in the control group (IPS 20) turned out to be 26.69 (± 7.99) MPa. The value in the IPS 40 group was 23.93 (± 8.08) MPa. The average adhesion in the IPS 60 group was 20.9 (± 7.91) MPa. Adhesive strength values in groups Eufar 20, Eufar 40, and Eufar 60 were 17.56 (± 6.61) MPa, 16.30 (± 11.68) MPa and 18.78 (± 10.22) MPa respectively.
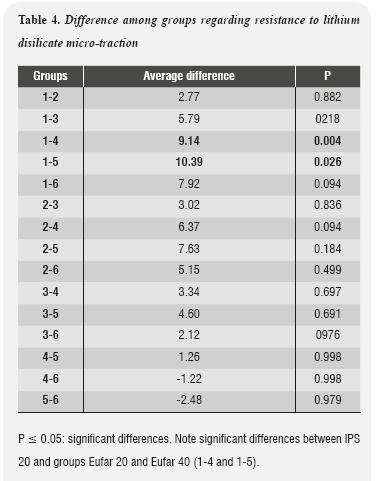
Results of the one-way Anova variance test suggested that at least one of the groups presents significant differences regarding lithium disilicate adhesion strength, since P values were 0.02. As this value was below 0.05, a Post Hoc analysis was performed in order to analyze the differences among groups. The results are shown in table 4.
DISCUSSION
Different in vitro methods to measure the bond strength of resin ceramic surfaces have been described, including tests of resistance to tension and shear forces. Concerning the latter, despite being the most commonly used for this type of study, their reliability is questioned because these tests do not always represent the real forces produced at the ceramic-resin interface.22, 23 Della Bona and Van Noort23 noted that adhesion strength of the ceramic-resin union is provided by the cohesive strength of the ceramics when measured by shear forces. This is due to high concentration of forces on this material at the site of force application, which produces a cohesive fault at very low compression values. On the other hand, tensile strength tests provide more representative data on adhesive strength at the union area, demonstrating an increase in adhesive failures when measuring.
Sano et al 18 developed the technique of micro- tensile bond strength, with the intention of assessing bond strength on small adhered areas. The authors showed that, if smaller adhesion areas are used, tensile strength bonding forces increase.
In our study, the obtained range of adhesion values coincides with the range reported by most authors using this technique. Spohr in 2003,8 Meyer in 2004,9 and Pollington in 2010,11 by using the micro- tensile bond strength test, obtained similar adhesion values, using different protocols of preparation of lithium disilicate ceramics.
The results of our study suggest that treating lithium disilicate surfaces with 4.6% hydrofluoric acid during 20 s, as a control group, provided the best performance, with an average of 26.69 (± 7.99) MPa of adhesion to a resinous substrate. This value presented significant differences compared to Group 4, with a discrepancy of 9.14 MPa and a value of P ≤ 0.05 (0.004), as well as with Group 5, which showed a difference of 10.39 MPa and P > 0.026. In the other groups, hydrofluoric acid concentration and time of application did not significantly affect adhesive strength during the micro-tensile bond strength test.
There are numerous techniques that seek to improve the values of ceramics' adhesive strength to its substrate. In their 2003 literature review, Blatz et al2 reported that the high content of lithium disilicate crystalline phase may produce higher adhesion values than those of leucite or feldspathic ceramics, regardless of the surface treatment these materials are subjected to. This may explain the similar results obtained among the groups of this study, with no significant difference in most of them regardless of the surface treatment used.
Other studies, such as those of Kiyan et al in 2007,7 Gökçe et al in 2007,10 and Kim et al in 2005,12 despite using conventional tensile strength techniques, confirm the effect of hydrofluoric acid concentration and time of application on the values of adhesion to a resinous substrate. So higher concentrations and application times result in a tendency to decreasing adhesion values on this type of materials.
Our study showed that etching with 4.6% hydrofluoric acid during 20 s provided the best results in terms of lithium disilicate adhesive strength, followed by the same concentration but with a time of application of 40 and 60 s. It also showed a decrease in adhesion values when increasing the etching agent's concentration and time of application.
These results provide clinical parameters to be considered during the process of cementation of lithium disilicate restorations. The maximum adhesion values confirm etching lithium disilicate surfaces with 4.6% hydrofluoric acid for 20 s and silane application as the golden rule in adhering this type of restorations.
We recommend to conduct further studies to correlate the obtained adhesion values by testing the tensile micro-strength of lithium disilicate adhered to a resinous substrate, as well as the material's surface characteristics observed in a scanning electron microscope, in order to provide greater foundations to the effect of concentration and time of application on the adhesion values in restorations made on lithium disilicate and cemented with resinous materials.
CONCLUSIONS
Even with the limitations of this study, we can conclude that:
The highest lithium disilicate adhesive strength averages, coupled with a resinous substrate, were obtained in groups that were treated with 4.6% hydrofluoric acid, compared with those etched at a concentration of 9.6%.
The average adhesive strength values obtained in our study may represent clinically acceptable values for a good clinical performance, according to previous studies.
CONFLICTS OF INTEREST
The authors declare having no conflicts of interest.
REFERENCIAS
1. Guess PC, Schultheis S, Bonfante EA, Coelho PG, Ferencz JL, Silva NR. All-ceramic systems: laboratory and clinical performance. Dent Clin North Am 2011; 55(2): 333-352. [ Links ]
2. Blatz MB, Sadan A, Kern M. Resin-ceramic bonding: a review of the literature. J Prosthet Dent 2003; 89(3): 268- 274. [ Links ]
3. Manso AP, Silva NR, Bonfante EA, Pegoraro TA, Dias RA, Carvalho RM. Cements and adhesives for all-ceramic restorations. Dent Clin North Am 2011; 55(2): 311-332. [ Links ]
4. Valandro LF, Della Bona A, Bottino MA, Neisser MP. The effect of ceramic surface treatment on bonding to densely sintered alumina ceramic. J Prosthet Dent 2005; 93(3): 253-259. [ Links ]
5. Zortuk M, K Kilic, Gurbulak AG, Kesim B, Uctasli S. Tensile bond strength of a lithium-disilicate pressed glass ceramic to dentin of different surface treatments. Dent Mater J 2010; 29(4): 418-424. [ Links ]
6. Della Bona A, Anusavice KJ, Mecholsky JJ Jr. Failure analysis of resin composite bonded to ceramic. Dent Mater 2003; 19(8): 693-699. [ Links ]
7. Kiyan VH, Saraceni CH, Silveira BL, Aranha AC, Eduardo CP. The influence of internal surface treatments on tensile bond strength for two ceramic systems. Oper Dent 2007; 32(5): 457-465. [ Links ]
8. Spohr AM, Sobrinho LC, Consani S, Sinhoreti MA, Knowles JC. Influence of surface conditions and silane agent on the bond of resin to IPS Empress 2 ceramic. Int J Prosthodont 2003; 16(3): 277-282. [ Links ]
9. Meyer FA, Clovis CL. Effect of different ceramic surface treatments on resin microtensile bond strength. J Prosthodont 2004; 13(1): 28-35. [ Links ]
10. Gökçe B, Özpinar B, Dündar M, Çömlekoglu E, Sen BH, Güngör MA. Bond strengths of all-ceramics: acid vs laser etching. Oper Dent 2007; 32(2): 173-178. [ Links ]
11. Pollington S, Fabianelli A, Van Noort R. Microtensile bond strength of a resin cement to a novel fluorcanasite glass-ceramic following different surface treatments. Dent Mater 2010; 26(9): 864-872. [ Links ]
12. Kim BK, Bae HE, Shim JS, Lee KW. The influence of ceramic surface treatments on the tensile bond strength of composite resin to all-ceramic coping materials. J Prosthet Dent 2005; 94(4): 357-362. [ Links ]
13. Brentel AS, Ozcan M, Valandro LF, Alarça LG, Amaral R, Bottino MA. Microtensile bond strength of a resin cement to feldpathic ceramic after different etching and silanization regimens in dry and aged conditions. Dent Mater 2007; 23(11): 1323-1331. [ Links ]
14. Hooshmand T, Van Noort R, Keshvad A. Bond durability of the resin-bonded and silane treated ceramic surface. Dent Mater 2002; 18(2): 179-188. [ Links ]
15. Matinlinna JP, Vallittu PK. Bonding of resin composites to etchable ceramic surfaces -an insight review of the chemical aspects on surface conditioning. J Oral Rehab 2007; 34(8): 622-630. [ Links ]
16. Aida M, Hayakawa T, Mizukawa K. Adhesion of composite to porcelain with various surface conditions. J Prosthet Dent 1995; 73(5): 464-470. [ Links ]
17. Kamada K, Yoshida K, Atsuta M. Effect of ceramic surface treatments on the bond of four resin luting agents to a ceramic material. J Prosthet Dent 1998; 79(5): 508-513. [ Links ]
18. Sano H, Shono T, Sonoda H, Takatsu T, Ciucchi B, Carvalho R et al. Relationship between surface area for adhesion and tensile bond strength -evaluation of a micro-tensile bond test. Dent Mater 1994; 10(4): 236-240. [ Links ]
19. Burrow MF, Thomas D, Swain MV, Tyas M J. Analysis of tensile bond strengths using Weibull statistics. Biomaterials 2004; 25(20): 5031-5035. [ Links ]
20. Pashley DH, Carvalho RM, Sano H, Nakajima M, Yoshiyama M, Shono Y et al. The microtensile bond test: a review. J Adhes Dent 1999; 1(4): 299-309. [ Links ]
21. Arias VG, Ambrosano GMB, Pimenta LAF. Determination of the sample and plot size for microtensile testing. J Dent Res 2006; 85: 8-18. [ Links ]
22. Van Noort R, Noroozi S, Howard IC, Cardew G. A critique of bond strength measurements. J Dent 1989; 17(2): 61-67. [ Links ]
23. Della Bona A, Van Noort R. Shear vs. tensile bond strength of resin composite bonded to ceramic. J Dent Res 1995; 74(9): 1591-1596. [ Links ]











 text in
text in