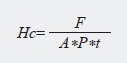Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Odontología Universidad de Antioquia
Print version ISSN 0121-246X
Rev Fac Odontol Univ Antioq vol.26 no.2 Medellín Jan./June 2015
ARTÍCULOS ORIGINALES DERIVADOS DE INVESTIGACIÓN
COMPARISON OF DENTINAL HYDRAULIC CONDUCTANCE ACCORDING TO THE APPLICATION TIME OF OXALATE-BASED DESENSITIZERS1
María de los Ángeles Romero2; Cristian Bersezio3; Patricio Vildósola4; Claudia Lletelier5; Osmil Batista Oliveira Jr.6; Javier Martín7; Eduardo Fernández7
1 This study was conducted within PRI-ODO 0304/2012 UCHILE. This
research project was partially funded by a Colgate-Palmolive-Chile
Grant; the authors report not having any conflicts of interest
2 Dental Surgeon, Universidad de Chile
3 Scholar, Department of Restorative Dentistry, Universidad de Chile.
4 Assistant Professor, Department of Restorative Dentistry, Universidad
de Chile
5 Assistant Professor, Department of Restorative Dentistry, Universidad
de Chile
6 Livre-docência Professor, Department of Restorative Dentistry, Dental
Area, Universidade Estadual Paulista, São Paulo, Brazil
7 Assistant Professors, Department of Restorative Dentistry, Universidad
de Chile. E-mail: edofdez@yahoo.com
SUBMITTED: NOVEMBER 19/2013-ACCEPTED: AUGUST 19/2013
Romero MA, Bersezio C, Vildósola P, Letelier C, Oliveira Jr OB, Martín J et al. Comparison of dentinal hydraulic conductance according to the application time of oxalate-based desensitizers. Rev Fac Odontol Univ Antioq 2015; 26(2): 336-357.
ABSTRACT.
INTRODUCTION: an in vitro model was used to measure the hydraulic conductance in human dentin discs treated with oxalic acid for 15, 30 or 60 s maintaining the occlusive effect and measuring 7 and 14 days after application.METHODS: 45 dentin discs measuring 1 mm thick were obtained from human third molars which were free of caries and in no occlusion; the samples were obtained from patients aged 16 to 30 years. Discs were sorted out into three study groups (n = 15) depending on the time of application of a commercial solution of oxalate-based dentin desensitizer (DD) (BisBlock®) which contains < 5% oxalic acid of 1.5-1.8 pH: in group A the agent was applied for 15 s, in group B it was applied for 30 s, and in group C for 60 s. The hydraulic conductance of each disc was calculated after acid etching, which corresponds to the maximum permeability of discs (100%) after immediate application of oxalic acid, as well as seven and fourteen days of storage in saline solution. The statistical analysis was done with ANOVA test and post-hoc Games-Howell test.
RESULTS: 35,46 ± 23.41% in Group A, 36.34 ± 15.88% in Group B and 24.99 ± 14.99% in Group C, showing that the use of DD for 15, 30 or 60 s decreased permeability in a statistically significant manner (p < 0.05).
CONCLUSIONS: DD was effective in reducing hydraulic conductance regardless of application time, but this reduction was temporary only, since after seven days permeability returns to values close to those of baseline.
Key words: dentin, conductance, diffusion, oxalic acid, permeability, dentin tubule occlusion.
INTRODUCTION
Dentin forms the central structure of teeth. It is crossed by tubules extending from the pulp to the enamel dentin junction. These tubules provide dentin with the physical feature of permeability.1
The permeability of a material can be defined as the capacity to allow the passage of solvent or solution through it. A material can be totally permeable to water, while solutes can or cannot pass through it. This property is assessed through hydraulic conductance, which is defined as the ability of a material to allow the passage of distilled water through it.1
The first description of substance permeability through dentin was published by Fritsch in 1914. In the 1940s, Lefkowitz observed that a dying agent injected in a tooth’s pulp penetrated the entire dentin in a little more than half an hour. A few years later, Bodecker and Lefkowitz2 observed that the coloring of a filling material applied on a deep cavity was able to move to adjacent areas of enamel, dentin, and pulp. These studies paved the way for the concept of "dentin permeability", currently defined as the passage of fluids, ions, molecules, particles, and bacteria in and through dentin under different conditions.
Several authors have studied the relationship of dentin permeability with its topography and morphology, and with different solutions.2, 3 The permeability of various components of dental materials and its possible cytotoxic effect on pulp cells has also been studied. Another frequent research field on dentin permeability consists on adhesive systems sealing tests. Due to this tissue’s complex hydration and nature, dentin adhesion and sealing of the exposed dentin surface remains a problem despite advances in adhesive dentistry. Permeability models have been used to calculate the capacity of adhesive systems to seal dentin and to determine the strength of adhesion of different adhesive systems, cements, and other dental materials to it.4-7
Many studies have reported that the application of various oxalate-based agents results in reduced dentin permeability ranging from 75 to 98.4%.8, 9Many studies have reported that the application of various oxalate-based agents results in reduced dentin permeability ranging from 75 to 98.4%.10 proved that the product with the lowest pH shows the greatest reduction in leakage rate and is less susceptible to the pre-treatment conditions of dentine. They concluded that the most acid gel was probably capable of releasing more calcium from dentin to react with potassium oxalate and form insoluble calcium oxalate crystals.
In order to prolong the occlusive effect of calcium oxalate, it has been suggested to use a protocol consisting in the application of oxalatebased desensitizers on etched dentin, with prior application of the bonding agent.
Based on the available information, we can state that, in vitro, oxalates have the potential to decrease permeability but only for a limited time. The influence of the agent’s application time is an unclear aspect of these compounds. By using an oxalate-based solution, Pereira et al established that the decrease in hydraulic conductance of non-etched dentin occurs 10 s after applying the solution, while in etched dentin the decrease is significant 60 s after application. In both groups, the size of precipitated crystal and the thickness of the layer of crystals increase with application time. In vitro and clinical studies evaluating oxalatebased agents show wide variations in application times, ranging from 15 s to 5 min, depending on the oxalic acid presentation.10
A clear example of this is what happens with 3% potassium oxalate monohydrate, which has been used in various in vitro studies on hydraulic conductance by applying it for 2, 3 or 4 minutes.4, 9, 10 All three studies found out that the use of this formula decreased hydraulic conductance in a statistically significant manner. This means that while application times are variable, the results of the in vitro studies are similar, with a statistically significant difference in terms of hydraulic conductance decrease following the application of oxalates.
The purpose of this study was to use an in vitro model to determine the hydraulic conductance of human dentin discs treated with oxalic acid for 15, 30 or 60 s maintaining the occlusive effect and measuring 7 and 14 days after application, using the basic protocol of application of commercial dentin desensitizing solution without subsequent application of an adhesive.
METHODS
This prospective experimental study included 45 human decay-free, in no-occlusion third molars extracted from volunteers aged 16 to 30 years, with a coronal diameter not greater than 12 mm. They were obtained once patients signed an informed consent approved by the Ethics Committee of Universidad de Chile School of Dentistry. The samples were disinfected for 24 hours in 0.1% Thymol solution (SIGMA, England), which is the permitted concentration, under the strict protocol and with no contact with the operators’ mucous membranes.11 They were later stored in 0.9% saline solution until use.12
The teeth were etched with 35% orthophosphoric acid (Coltene-Whaledent) all through the enamel for 30 seconds in order to remove post-attrition dentinal smear layer. Then they were washed, dried, and applied a layer of cyanoacrylate. Once dry, the pieces were embedded in epoxy resin blocks with a cylindrical shape of 13 mm in diameter and 20 mm high, manufactured in silicone mold, until polymerization was completed.
Samples were sectioned with a low-speed 1000 Isomet® saw (ISOMET BUEHLER LAKEBUFF IL, USA 1000, 750r/min, 250 g) under abundant refrigeration, parallel to the occlusal face of teeth, in order to obtain 1 mm thick dentin discs. Once discs had been sectioned, their occlusal sides were polished with sandpaper (n. 600 Silicon carbide) under running water to standardize remnants of dentinal smear layer. Each disc was labeled pointing out its occlusal face and indicating the group to which it belonged; the discs were randomized using the SPSS 21.0 software.
Each disc’s occlusal and pulpal dentin was etched with 35% orthophosphoric acid (Coltene- Whaledent), for 15 s to remove dentinal smear layer following the manufacturer's "basic technique" protocol, and then washed with water for 30 s.
achieve an experimental model consistent with the clinical conditions of dentin sensitivity, in which dentin has open dentin tubules, permeability was measured after removal of dentinal smear layer. The hydraulic conductance obtained under these conditions can be considered as the maximum permeability of each disc, i.e. 100%; therefore, all the hydraulic conductance measurements later made on the same disc (after application of oxalic acid and 7 and 14 days of application), were considered as a percentage of this 100%. Thus, and taking into account that each disc is unique in terms of its tubules density, each disc acted as its own control. The dentin desensitizing agent used was the commercial brand BisBlock® (Bisco Inc, Schaumburg, IL, USA).
Study groups
3 groups of 15 dentin discs each were formed:
- Group 1: 15 dentin discs previously etched with 35% orthophosphoric acid, which were applied oxalic acid with a microbrush (BisBlock®;, Bisco), letting it act for a period of 15 s, and washed with abundant water.
- Group 2: 15 dentin discs previously etched with 35% orthophosphoric acid, which were applied oxalic acid with a microbrush (BisBlock®, Bisco), letting it act for a period of 30 s, and washed with abundant water.
- Group 3: 15 dentin discs previously etched with 35% orthophosphoric acid, which were applied oxalic acid with a microbrush (BisBlock®, Bisco), letting it act for a period of 60 s, and washed with abundant water.
- Negative control: epoxy resin disc.
Once treated with oxalic acid, samples were stored at room temperature (25° C) and with controlled humidity (100%) in the FOUCH Chemistry Lab. The samples’ leakage was later measured in the following time intervals:
- Immediately after application.
- After 7 days.
- After 14 days.
Each measurement was made for a period of 20 minutes and was repeated 3 times in order to get average data. Once measurements were completed, four hydraulic conductance values were obtained for each dentin disc:
- Initial value: analysis of dentin disc with acid etching only.
- Final values: analysis of dentin disc etched and treated with an oxalate-based agent during certain application time which was measured at three different time intervals.
The experimental model used was validated by Hevia et al,13 and is similar to that of Reeder et al.14 It was modified by removing the thermostat it includes, since temperature is not a variable to be studied in our work. This equipment enabled to determine the rate of filtration through dentin, i.e., the volume of leaked fluid per unit of time. It consisted of a reservoir with a vertical column of water of 20 cm connected to a stopcock which, by means of a silicone tube, was connected to a capillary graph in a horizontal position which has a capacity of 0.1 ml. This pipette was supplied by one end with an air bubble which functioned as a viewing guide and later as a measurement of leakage through dentin. The capillary was distally connected to a chamber where the dentin discs under study were fixed by means of a silicone tube. The chamber where discs were placed was modified in order to observe any leakage occurring between tooth and resin. To this end, the upper part of the chamber was removed with a carbide bur. Thus, prior to beginning each measurement, the operators made sure that water leaked through dentin only. When discs had leakage, the areas were marked and later filled with cyanoacrylate. Once leakage speed values had been obtained, hydraulic conductance was calculated with the following formula:
Hc: dentin hydraulic conductance in µl/cm2/min, F: filtration rate in µl/min., A: dentin surface area in cm2, P: difference of hydrostatic pressure through dentin in cm H2O, t: measurement time in min.
Hydraulic conductance corresponds to a formula that determines the permeability of dentin discs (in this case). The objective was to calculate this equation in each group. Variable F corresponds to each disc’s fluid rate. Variable A corresponds to the dentin area exposed to the fluid. Variable P corresponds to the intra-pulpal pressure whose value corresponds to the height of the column of distilled water (20 cm), and variable T is the measurement time—20 min in this case—. The area of exposed dentin per disc was calculated by the ImageJ,15 computational software by means of previous photographic records. This is a digital image processing software that allows calculating certain area by counting the number of pixels it has.
Statistical analysis: data distribution and homogeneity was tested (Shapiro Wilk Test and Levene Test) and compared using ANOVA test and post-hoc Games-Howell test. This was the same for all comparisons. Furthermore, the G*Power 3.1 software was used to define the sample with p = 0.05 confidence interval, 0.83 statistical power, and 0.5 effect size.
RESULTS
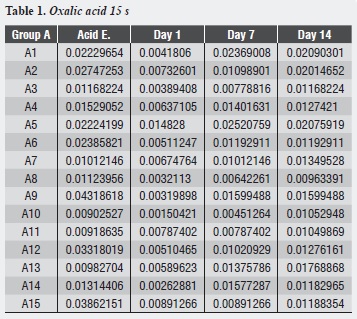
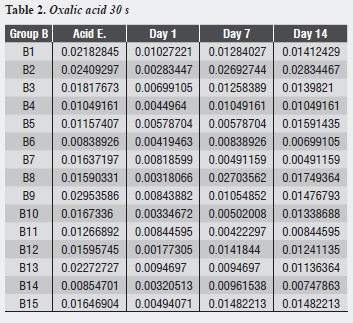
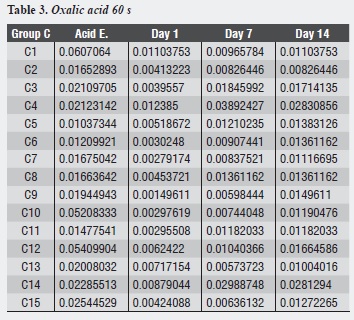
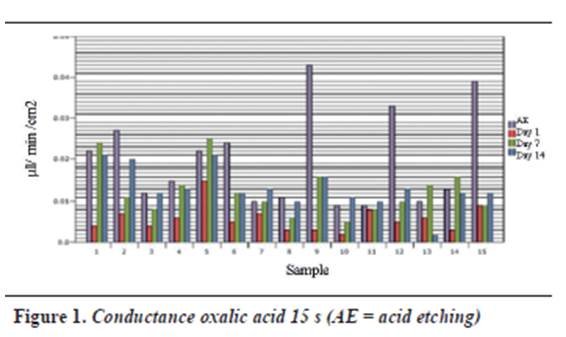
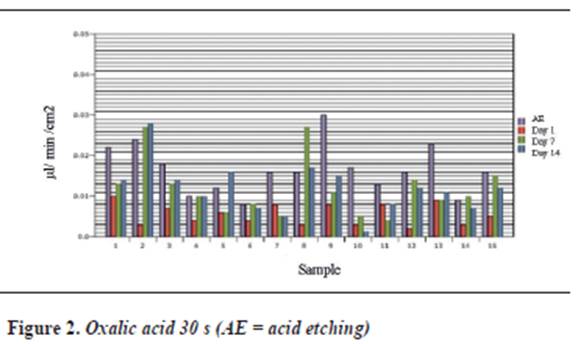
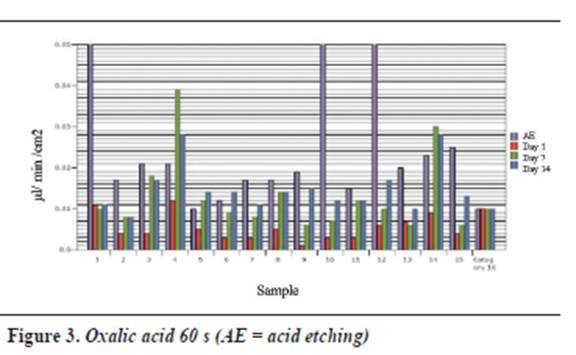
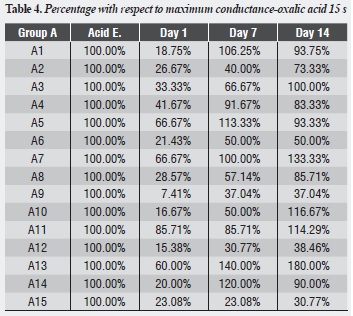
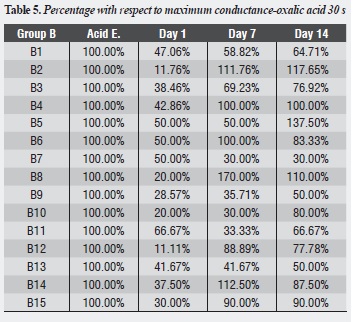
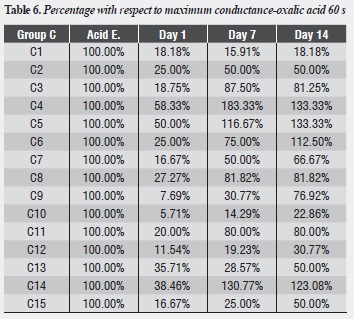
Table 1, table 2 and table 3 and figure 1,figure 2 and figure 3 show each dentinal disc’s hydraulic conductance values in each evaluation time for groups A, B and C respectively. In addition, parentheses show conductance expressed as a percentage of maximum conductance (100%) of each disc (following acid etching) (table 4, table 5 and table 6).
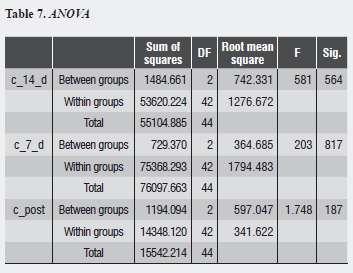
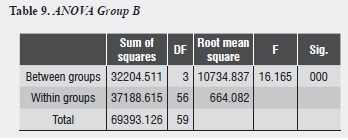
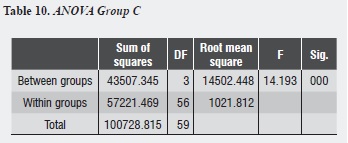
There was no statistically significant difference in terms of conductance percentages with respect to maximum filtration among the study groups. While in the three evaluation times (immediately, 7 days later, and 14 days later) Group C was the one with the lowest conductance values, there was no significant difference among the three groups immediately after oxalic acid application (p = 0.187), 7 days afterwards (p = 0.817), or 14 days later (p = 0.564) (table 7).
There is no difference among the groups immediately after application (p = 0.187), 7 days afterwards (p = 0.817), or 14 days later (p = 0.564).
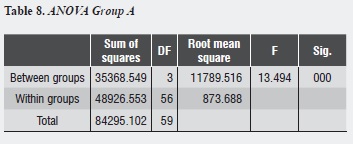
Group A shows that the largest decrease in hydraulic conductance (64.54%) occurs immediately after treatment with oxalic acid, and this decrease is statistically significant (p = 0.000). After one week of treatment, conductance begins to increase, being statistically significant (p = 0.011). Between days 7 and 14, while a new increase in conductance occurs, there is no statistically significant difference between them (p = 0.758). There is no difference between the post-etching value and the value 7 and 14 days later (p = 0.07, p = 0.661) respectively (table 8).
Group B shows that the largest decrease in hydraulic conductance (63.66%) occurs immediately after treatment with oxalic acid, and this decrease is statistically significant (p = 0.000). After one week of treatment, conductance begins to increase, being statistically significant (p = 0.014). Between days 7 and 14, while a new increase of conductance occurs, there is no statistically significant difference between them (p = 0.951). There is no difference between the post-etching value and the value 7 and 14 days later (p = 0.118, p = 0.089) respectively (table 9).
Group C shows that the largest decrease in hydraulic conductance (75.01%) occurs immediately after treatment with oxalic acid, and this decrease is statistically significant (p = 0.000). After one week of treatment, conductance begins to increase, being statistically significant (p = 0.031). Between days 7 and 14, while a new increase of conductance occurs, there is no statistically significant difference between them (p = 0.958). There is no difference between the post-etching value and the value 7 and 14 days later (p = 0.074, p = 0.081) respectively (table 10).
DISCUSSION
The measurement of changes in permeability or fluids flow through dentin has been frequently used to assess the sealing ability of adhesive and non-adhesive restorative materials, the toxic potential of dental materials, the effectiveness of toothpastes and desensitizing materials, the absorption of substances, or the effect of various clinical procedures.16 This study was based on the method used by Reeder et al14 in permeability studies characterized by simple, effective, and economic systems.
The goal of treatment with desensitizing agents is to reduce hydraulic conductance to values close to 10-20% (of total permeability),9 such as those obtained with dentinal smear layer, since many studies report that its presence is responsible for 80- 85% of the resistance to fluid flow through dentin.10 The conductance values obtained after oxalic acid application were between 24 and 36% of maximum permeability, which even though are higher than the expected range are statistically significant so they could be considered as a good predictor of the occlusive power of the studied material.
The application of this commercial solution of dentinal desensitizer proved to be effective in reducing dentinal permeability when applied on previously-etched dentin, with a statistically significant reduction (p < 0.05), which is consistent with conductance studies that evaluate oxalatebased agents. 4, 10, 17, 18 This efficacy has been proven in dentin with intact smear layer,4 where it has been shown to be replaced with a layer of crystals, as well as in previously-etched dentin with subsurface formation of crystals.
In a permeability study using the same commercial solution of dentin desensitizer, Yiu et al19 reported that the percentage of conductance reduction is even higher than the one obtained with a series of adhesive agents. The percentage of reduction of hydraulic conductance following the application of oxalic acid with our commercial solution of dentin desensitizer ranged from 63 to 75% in our study—values slightly lower than the percentages described in the literature, which range from 75 to 98.4%—.9, 10, 18-20 Yiu et al19 reported that hydraulic conductance reduction following the application of the agent for 30 s on etched dentin was between 88 and 91%. However, even though the percentage obtained in the present study is slightly lower, it was possible to establish that the permeability reduction in the three study groups immediately after the application of oxalic acid is statistically significant with respect to the disc’s maximum permeability.
These differences in reduction percentage between the literature reports and the present study could be due to differences in the studies’ methodologies or to deficiencies of the agent used.2 From the methodological point of view, this project presents some differences with the studies already published. The system needs some pressure for the bubble to set in motion. This study used a pressure of 20 cm H2O, which is close to the physiological pressure of dental pulp (± 14 cm H2O) but much lower than the pressure used by many studies that range between 700 and 1050 cm H2O 4, 9, 10, 18 These values are used to have faster reading speeds, since the experiments carried out with reduced pressure experience reading delays and operator fatigue because of the bubble moving very slowly.18
In the experimental model we used, pressure is determined by the height of the column of water, unlike other studies that use nitrogen, allowing higher pressure levels. However, the used pressure loses relevance since the mathematical hydraulic conductance formula standardizes the results. However, it has been described that the amount of applied pressure and application time can somehow interfere with hydraulic conductance measurements.21 An increase in flow resistance can occur under great pressure, over 53.3 KPa (551.3 mmH2O), due to compression of intratubular content against the walls of the tubules, reducing hydraulic conductance values, while physiological pressure does not affect the tubular content providing results closer to those that occur in vivo.21
On the other hand, the published studies use a standard filtration area that is the same for all discs, which is obtained by using a pair of rubber rings with equal dimensions placed on the discs’ pulp side and the occlusal side.4, 18, 19, 22, 23 This system of area measurement was not used since it does not consider the regional variability of dentin permeability. Measurements in the center of discs (which is a low permeability area) usually produce overestimation of hydraulic conductance since the rubber rings overlap the more permeable areas—that of the pulp horns—. 24 In this study, area was calculated by ImageJ,15 a computer software which requires manual delimitation of the enamel dentine junction. This system does not allow determining very small variations in fluid flow, which is determined by capillary volume.16 The volume of the micro pipette we used showed that the minimal permeability variation we could detect was 0.001 ml, which corresponds to the difference between a calibration mark and the other, which impedes the accurate detection of any permeability changes lower than this value.
In contrast to the present study, Yiu et al19 used the FLODEC system to measure conductance; in that system, evaluation of the bubble’s movement is automatic and thanks to the capillary graph design it can detect small changes in fluid flow16. It has been shown that results obtained using these two methods are interchangeable, although they are not identical.16 Concerning the agent used, its effectiveness is mainly related to pH and solution concentration as they are relevant in terms of the amount of crystals they precipitate. According to the manufacturer, the commercial solution of dentin desensitizer has a pH ranging from 1.5 to 1.8, which is slightly lower than that of the agents used in other studies, whose pH ranges from 2 to 4.4, 10, 23 Since the literature reports that the more acid the gel the greater capacity it will have to release calcium from dentin to react with the oxalate ions, we expected that this commercial solution of dentin desensitizer would be capable of releasing a sufficient amount of calcium to react with oxalic acid.
On the other hand, the size of the precipitated crystal is thought to be related to the concentration of agent—a condition that could affect the occlusive power of the formulation, demonstrating that a 30% potassium oxalate-based solution causes crystals of much bigger size than those produced by a 3% solution.4, 25 SEM has shown that this commercial solution of dentin desensitizer produces crystals of angular appearance which on etched dentin are located in the interior of tubules at a distance of 10-15 µm from the dentin surface, blocking the tubules and their lateral branches.19 However, the manufacturer does not provide a specific concentration of oxalic acid but rather a range of < 5%, which could explain the difference in permeability reduction percentages, since the concentration could have been very low. The molar concentration of oxalic acid was 3.17 x 10-2, which is equivalent to a percentage of w/v = 0.285 and is much lower than that used in other studies, ranging from 3 to 6%. However, it should be taken into account that this is only a reference calculation made in this study.
The ANOVA test showed that there is no statistically significant difference (p > 0.05) in hydraulic conductance reduction following application of oxalic acid for 15, 30 or 60 s in none of the three measurement times. This is consistent with the available information in that, while the same formulations of oxalic acid are used, it is done at different application times and with similar results, as happens with 3% potassium oxalate monohydrate, pH 2.5 or 4, which is one of the most widely used in the studies.
Pereira et al9 measured hydraulic conductance following the application of it for 4 min; Santiago et al18 used it on dentin surfaces for 3 min, while Pashley et al4 did it for only 2 min. These three studies showed that the use of this formula decreases hydraulic conductance in a statistically significant manner with respect to the discs’ maximum permeability, regardless of application time.
A recent literature review concludes that this is effective in the treatment of dentin sensitivity, even though the included studies use application times ranging from 15 s to 3 min.26 Jain et al11 used ferric oxalate on dentin discs, showing that an increase in permeability starts to happen after one day of immersion in artificial saliva, but this was not statistically significant. Later, Suge et al27 measured the duration of dentinal tubules occlusion by using a potassium oxalate-based solution, concluding that after 7 days of storage in artificial saliva there is a gradual and steady increase of permeability and this immersion generates a high concentration of oxalate ions in the medium, indicating dissolution of calcium oxalate.
SEM analysis confirms that after seven days of exposure to the oral environment, the surface shows few crystals on the dentin surface and the tubules appear once again open. The solubility of calcium oxalate is important to determine the duration of dentinal tubules occlusion. Although it has been suggested that they form layers of resistant acid crystals when reacting with calcium from the dentin,4, 10, 23 other studies argue that calcium oxalate crystals are acid-labile and can be easily removed from the dentin surface, suggesting that the solubility of calcium oxalate is sensitive to pH changes since its anion is the conjugate base of a weak acid.19
According to Le Chatelier’s principle, when calcium oxalate crystals are exposed to H3O+ it dissociates into calcium and oxalate ions to compensate for the depletion of ions oxalates produced by the formation of oxalic acid and thus maintain a constant balance.19 It is likely that, because of this, occlusion in the present study was of a short duration, since the saline solution used as storage medium has a pH between 5.5 and 7, slightly acid, which could generate dissolution of calcium oxalate crystals. When a crystal such as calcium oxalate is stored in a solution, there is a balance between both mediums: the solid (glass) and the liquid phase. If calcium oxalate is immersed in artificial saliva, the partial dissolution of calcium oxalate brings the solution to a balance with regard to it. In the oral environment, calcium oxalate would continuously find fresh saliva, which does not contain oxalate ions, so its dissolution would continue slowly but steadily until reaching a new state of balance.27 Furthermore, the solubility of this ionic compound changes if the solution becomes sufficiently acidic or basic.
Oxalate C2O4)-2 is the conjugate base of oxalic acid (H2C2O4), therefore, when the medium is acidified it produces protonation of anion to form the acid. When oxalate is decreased, the system tends to replenish by dissolving more calcium oxalate. We will take this example as a basis, since other salts with basic anions such as oxalate(C2O4)-2,behave similarly.
It has been showed that the solubility of poorly soluble salts containing basic anions increases as pH decreases. This could explain why oxalates experience a sensitivity reduction which is only temporary, since the normal pH of saliva is 6 to 7, slightly acidic, with fluctuations between 5.3 and 7.8,28 which would contribute to speed up these crystals dissolution in the mouth. Dentinal permeability decrease is important in the treatment of sensitivity since according to the hydrodynamics theory the occlusion of dentinal tubules decreases the flow of fluids inside them in the presence of stimuli that cause pain.
As shown in this study, oxalates have this occlusion ability by reducing hydraulic conductance; however, the hydraulic conductance increase until day seven suggests a loss of such tubular occlusion, which would bring back sensitivity during this period, disagreeing with the clinical findings that show that the commercial solution of dentin desensitizer was effective in reducing sensitivity for a period of up to 4 weeks.8, 29 However, it should be noted that those studies used an adhesive layer following application of the agent, which would help slow down the loss of crystals, which was not an objective of the present study. 30
Even though the occlusion produced by calcium oxalate crystals appears to be brief, it has been suggested that applying these agents can reduce dentin sensitivity before manifestation of the natural occlusion of tubules from minerals in the saliva, which seems to take place within 28 days after tubules exposure.31
Limitations of the present study include not using an adhesive, as indicated by the manufacturers, after applying oxalate-based dentin desensitizer solutions. However, this study sought to understand the effect of solution application time in order to be able to control this variable, by explaining crystals dissolution in a controlled environment and to find a probable state of lower concentration of crystals in exposed discs due to storing over time.
CONCLUSIONS
- Application of oxalic acid in a commercial solution of dentin desensitizer for 15, 30 or 60 s significantly reduces hydraulic conductance to values close to the original dentinal smear layer, according to the experimental conditions of this study, where dentin adhesive was not subsequently used.
- Oxalic acid application times do not affect hydraulic conductance values immediately after application, nor 7 or 14 days later.
- After seven days of storage, there is a statistically significant increase in hydraulic conductance, which reaches levels similar to maximum permeability after acid etching, under the experimental conditions of our study.
CONFLICT OF INTEREST
The authors declare not having conflicts of interest.
REFERENCES
1. Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci 2011; 3: 711-735. [ Links ]
2. Bodecker CF, Lefkowitz W. Further observations on vital staining of dentin and enamel. J Dent Res 1946; 25(5): 387-399. [ Links ]
3. Vachiramon V, Vargas MA, Pashley DH, Tay FR, Geraldeli S, Qian F et al. Effects of oxalate on dentin bond after 3-month simulated pulpal pressure. J Dent 2008; 36(3): 178-185. [ Links ]
4. Pashley DH, Carvalho RM. Dentine permeability and dentine adhesion. J Dent 1997; 25(5): 355-372. [ Links ]
5. Pashley DH, Depew DD. Effects of the smear layer, Copalite, and oxalate on microleakage. Oper Dent 1986; 11(3): 95-102. [ Links ]
6. Soares DG, Ribeiro AP, Sacono NT, Coldebella CR, Hebling J, Costa CA. Transenamel and transdentinal cytotoxicity of carbamide peroxide bleaching gels on odontoblast-like MDPC-23 cells. Int Endod J 2011; 44(2): 116-125. [ Links ]
7. Lanza CR, de Souza Costa CA, Furlan M, Alécio A , Hebling J. Transdentinal diffusion and cytotoxicity of selfetching adhesive systems. Cell Biol Toxicol 2009; 25(6): 533-543. [ Links ]
8. Lessa FC, Nogueira I, Huck C, Hebling J, Costa CA. Transdentinal cytotoxic effects of different concentrations of chlorhexidine gel applied on acid-conditioned dentin substrate. J Biomed Mater Res B Appl Biomater 2010; 92(1): 40-47. [ Links ]
9. Erdemir U, Yildiz E, Kilic I, Yucel T, Ozel S. The efficacy of three desensitizing agents used to treat dentin hypersensitivity. J Am Dent Assoc 2010; 141(3): 285-296. [ Links ]
10. Pereira JC, Segala AD, Gillam DG. Effect of desensitizing agents on the hydraulic conductance of human dentin subjected to different surface pre-treatments--an in vitro study. Dent Mater 2005; 21(2): 129-138. [ Links ]
11. Jain P, Reinhardt JW, Krell KV. Effect of dentin desensitizers and dentin bonding agents on dentin permeability. Am J Dent 2000; 13(1): 21-27. [ Links ]
12. Andersen A. Final report on the safety assessment of sodium p-chloro-m-cresol, p-chloro-m-cresol, chlorothymol, mixed cresols, m-cresol, o-cresol, p-cresol, isopropyl cresols, thymol, o-cymen-5-ol, and carvacrol. Int J Toxicol 2006; 25 Suppl 1: 29-127. [ Links ]
13. Araya F, Sommariva C, Moncada G, Cartagena A, Letelier C, Oliveira O Jr et.al. Efecto del almacenamiento en solución de HBSS sobre la difusión transdentinaria en terceros molares extraídos. Rev Fac Odontol Univ Antioq 2013; 25(1): 158-175. [ Links ]
14. Hevia J, Fresno C, Martín J, Moncada G, Letelier C, Oliveira Junior OB et al. Modelo de conductancia hidráulica de la dentina humana ex vivo. Rev Clin Periodoncia Implantol Rehabil Oral 2013; 6(3): 114-117. [ Links ]
15. Reeder OW, Walton RE, Livingston MJ, Pashley DH. Dentin permeability: determinants of hydraulic conductance. J Dent Res 1978; 57(2): 187-193. [ Links ]
16. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9(7): 671-675. [ Links ]
17. De La Macorra JC, Escribano NI. Comparison of two methods to measure permeability of dentin. J Biomed Mater Res 2002; 63(5): 531-534. [ Links ]
18. Pashley EL, Tao L, Derkson G, Pashley DH. Dentin permeability and bond strengths after various surface treatments. Dent Mater 1989; 5(6): 375-378. [ Links ]
19. Santiago SL, Pereira JC, Martineli AC. Effect of commercially available and experimental potassium oxalate-based dentin desensitizing agents in dentin permeability: influence of time and filtration system. Braz Dent J 2006; 17(4): 300-305. [ Links ]
20. Yiu CK, Hiraishi N, Chersoni S, Breschi L, Ferrari M, Prati C et al. Single-bottle adhesives behave as permeable membranes after polymerisation. II. Differential permeability reduction with an oxalate desensitiser. J Dent 2006; 34(2): 106-116. [ Links ]
21. Greenhill JD, Pashley DH. The effects of desensitizing agents on the hydraulic conductance of human dentin in vitro. J Dent Res 1981; 60(3): 686-698. [ Links ]
22. Camps J, Martin P, Ladeque P, Rieu R, Fuseri J. Influence of tooth cryopreservation on human dentin permeability, in vitro. Dent Mater 1994; 10(3): 210-214. [ Links ]
23. Derkson GD, Pashley DH, Derkson ME. Microleakage measurement of selected restorative materials: a new in vitro method. J Prosthet Dent 1986; 56(4): 435-440. [ Links ]
24. Pashley DH. Dentin permeability, dentin sensitivity, and treatment through tubule occlusion. J Endod 1986; 12(10): 465-474. [ Links ]
25. Pashley DH, Andringa HJ, Derkson GD, Derkson ME, Kalathoor SR. Regional variability in the permeability of human dentine. Arch Oral Biol 1987; 32(7): 519-523. [ Links ]
26. Muzzin KB, Johnson R. Effects of potassium oxalate on dentin hypersensitivity in vivo. J Periodontol 1989; 60(3): 151-158. [ Links ]
27. Cunha-Cruz J, Stout JR, Heaton LJ, Wataha JC. Dentin hypersensitivity and oxalates: a systematic review. J Dent Res 2011; 90(3): 304-310. [ Links ]
28. Suge T, Ishikawa K, Kawasaki A, Yoshiyama M, Asaoka K, Ebisu S. Duration of dentinal tubule occlusion formed by calcium phosphate precipitation method: in vitro evaluation using synthetic saliva. J Dent Res 1995; 74(10): 1709-1714. [ Links ]
29. Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent 2001; 85(2): 162-169. [ Links ]
30. Pamir T, Kaya AD, Baksi BG, Sen BH, Boyacioglu H. The influence of bonding agents on the decision to replace composite restorations. Oper Dent 2010; 35(5): 572-578. [ Links ]
31. Yiu CK, King NM, Suh BI, Sharp LJ, Carvalho RM, Pashley DH et al. Incompatibility of oxalate desensitizers with acidic, fluoride-containing total-etch adhesives. J Dent Res 2005; 84(8): 730-735. [ Links ]
32. Kerns DG, Scheidt MJ, Pashley DH, Horner JA, Strong SL, Van Dyke TE. Dentinal tubule occlusion and root hypersensitivity. J Periodontol 1991; 62(7): 421-428. [ Links ]











 text in
text in