Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Odontología Universidad de Antioquia
Print version ISSN 0121-246X
Rev Fac Odontol Univ Antioq vol.26 no.2 Medellín Jan./June 2015
ORIGINAL ARTICLES DERIVED FROM RESEARCH
LIESEGANG RINGS AS ORIGIN OF ENAMEL SYMMETRICAL PATTERNS
Mario Rodríguez Blanco1; Edgar Delgado Mejía2
1 DDM, Specialist in Oral Rehabilitation, Candidate to MA in Dentistry,
Universidad Nacional de Colombia. E-mail: merodriguezb@unal.edu.co
2 Chemist, Master of Science (Chemistry), Associate Professor,
Department of Chemistry, Universidad Nacional de Colombia. E-mail: edelgadom@unal.edu.co
SUMBITTED: SEPTEMBER 10/2013-APPROVED: JULY 3/2014
Rodríguez M, Delgado E. Liesegang rings as origin of enamel symmetrical patterns. Rev Fac Odontol Univ Antioq 2015; 26(2): 447-467.
ABSTRACT
INTRODUCTION: teeth contain various visible structures that have repeating shapes and symmetrical patterns such as prisms, crests,
enamel spindles, Hunter-Schreger bands, and Retzius incremental lines. On the other hand, Liesegang rings, studied and applied for over a hundred
years by geologists and other specialists, are incremental repetitive symmetrical bands found in natural minerals which are similar to those
observed in tooth enamel. This article aims to review the widely known processes of formation of Liesegang rings in nature and relate them with
dentin mineralization and the conformation of their characteristic anatomy.
METHODS: to this end, a bibliographic review was conducted, restricted
to the 1970-2013 period, in the Science Direct, Springer, Medline, and Pubmed databases, finally selecting 51 references with original information
or relevant data on the subject.
RESULTS AND CONCLUSIONS: a detailed analysis of the processes of formation of these rings and the similarity of rocky
and dental minerals lead to think that the processes developed in rocks and hard dental tissues would be the same.
Key words: Liesegang rings, electrolytes, morphogens, dentin, physiological calcification.
INTRODUCTION
When the histology of the enamel is studied in detail, the description of the different components of its anatomy call our attention because of being repetitive symmetrical structures with rather constant shapes such as prisms, crests, enamel spindles, Hunter-Schreger bands, Retzius incremental lines, and perykimata. It is then logical to ask: What is the origin of these structures? Are they all the same? Why are there dark bands and transparent bands? Why do thickness and distance between the bands seem to gradually change with position? Is this set of bands the same for everyone or does it vary in each person? If so, could these bands be used for identification purposes? Would the positions and dimensions of these bands change with age? Can they serve to assess age?
Some fields apparently unrelated to dental issues have approached topics that can solve these questions. Being characteristic elements of different nature structures, Liesegang rings have been the subject of observation and analysis of many researchers since the end of the 19th century. During this period, the symmetrical patterns of rocks, seashells, tree stems, butterfly wings, bands of colors in animal fur, and other natural materials began to be studied, arousing the interest of the scientific community, and they have been explained and predicted by using the concepts of Liesegang rings.
The study of these patterns began with the empirical observations of Raphael Eduard Liesegang in 1896 and has been complemented by current mathematical modeling, which allows predicting the formation of sequential bands both in geological and biological fields. However, it seems that the implementation of these principles has not fully reached the dental area, where its use promises to be very fruitful.
This study highlights the physical and chemical processes involved in this phenomenon and interprets them within the events that determine the characteristic morphology of the enamel in teeth.
In order to review the processes of formation of these structures, it is necessary to go into some detail to learn the principles and terminology of Liesegang including the definition of rings, the original studies, the concept of supersaturation, the contribution of Alan Turing, mathematical biology, the process of dentin biomineralization, and its relationship with the formation of incremental bands.
METHODS
A bibliographic review restricted to the period between 1970 and 2013 in the Science Direct, Springer, Medline, and Pubmed databases was conducted using the following search terms: Liesegang rings, electrolytes, morphogens, tooth enamel, physiological calcification, and others such as Ostwald's supersaturation theory, embryology of tooth enamel, and dental mineralization. Reference books were used to obtain basic concepts on the subject.
As an exclusion criterion, studies with a purely geological or mathematical focus were not taken into account, exclusively analyzing the studies with chemical or biological components that allowed its association with the dental area. After reviewing 65 articles and books, 51 reference publications were selected.
RESULTS
Definition of Liesegang rings
There are two main definitions: the first one defines them as concentric, secondary bands or rings caused by the rhythmic precipitation of saturated fluids in rocks.1 The second definition states that they are oscillating reactions in time and space, in which conditions of the system's self-organization are periodically met.2, 3
Original research by Liesegang
Raphael Eduard Liesegang, a German chemist and photographer, was who, by experimenting in his photographic laboratory in 1896, noted this phenomenon by accidentally dropping potassium chromate in a silver nitrate solution.
He later reproduced the process in his laboratory as follows: he took 100 ml of distilled water and heated it to approximately 70 °C; then he dissolved 4 g of common gelatin for desserts by stirring the mixture and added 0.2 g of potassium chromate until its complete dissolution. Then he spread the transparent yellow solution, still hot, in a flat container in order to form a sheet of 2 to 3 mm thick. The solution was left to cool, and after a few hours, when the mixture became a gel, he poured on the surface a drop of a colorless transparent solution, consisting of 0.25 g of silver nitrate in 1 ml of distilled water.
In the next days, he observed that red concentric rings (silver chromate) developed around this drop, alternating with zones which retained the yellow color (potassium chromate).4-6
Ostwald's concept of supersaturation
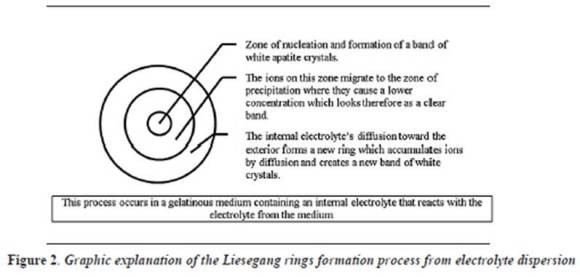
The research carried out at the time with different chemical compounds showed similar behaviors. However, there was no detailed scientific understanding of the underlying processes. Wilhelm Ostwald analyzed this phenomenon and suggested the theory of supersaturation to explain the formation7-9 of incremental bands in different elements of nature in chemical balance, chromatography, and catalysis,10, 11 and formulated the law of dilution or Ostwald Law, which explains the dissociation of electrolytes12 in solutions. Ostwald suggested that the formation of precipitates is not immediate and that a volume oversaturated with the reagent occurs first; when the reagent reaches the limit of stability, the precipitate is formed by nucleation of the product.
The term supersaturated indicates that the solution contains an excess of solute above what corresponds to normal solubility. The more excess in solute the solution contains, the more unstable it becomes. When it reaches maximum instability, the excess solute passes from being in a solution to separating as a solid, and for this to occur, nuclei of the insoluble solid form first and then grow. This localized nucleation decreases the concentration of the precipitate in adjacent areas, where a clear band appears caused by a lower concentration of precipitate. Chemical species slowly go through this space, increasing saturation in a new point and generating a new band as seen by microscopy in incremental enamel bands
In the experiment by Liesegang, the electrolytes are chromate, sodium, silver, and nitrate. In the case of hard tissues, the electrolytes are calcium and phosphate, which finally form biological apatites which are poorly soluble. The solubility of poorly soluble substances is measured as the constant of product solubility or Kps.
The result is not always the same since the rhythmic precipitations in Liesegang reaction are very sensitive to small fluctuations of variables such as temperature, pH, porosity of the gel, or dimensions of the container.
A slightly clearer explanation of the process is that a concentrated solution of a reactant A, called internal electrolyte, dissolves in a gelatinous medium, and then another compound or reactant B, called external electrolyte, spreads in the same medium causing a chemical reaction which in turn produces a third insoluble compound, which saturates the medium causing a nucleation process which hosts the formation of bands or rings well defined by the insolubility of the new compound in the environment (Liesegang rings). As this is an oscillating reaction in time with new chemical contributions, it offers the conditions for the formation of organized and sequential systems of rings.13 Within the gelatinous medium, a nucleation area is formed with a precipitation of ions around the site of contribution of electrolytes, causing a decrease in ionic concentrations in the medium that favors spreading of the internal electrolyte and starts the formation of a new ring.
Following this concept, we can easily conclude that the constitutive elements of the process and the way in which they interact determine the individual and unique characteristics of the formed bands.
A detailed study of such characteristics would make it possible to know the concentrations of reactants, the temperature at which the chemical process occurred, as well as its viscosity, water content, diffusion coefficients, and the history of formation of these structures, as the study of rings of tree stems has traditionally been.
Turing's contribution
The English mathematician Alan Mathison Turing (1912-1954),14 an expert in logics, computing, cryptography, and philosophy, discovered several factors that had not been clearly explained, such as the emergence of irregular spiral patterns, the presence of particles suspended within and between rings—which are now easily detected with modern techniques—, and discontinuities in the distribution of precipitate particles.
Based on Ostwald's dissociation phenomena, Turing developed crucial equations in pattern formation. With his works on Mathematical Biology (the modeling of biological processes from mathematical equations),15 particularly in morphogenesis, he published the book The Chemical Basis of Morphogenesis in 1952.
Incremental bands formation patterns, whether they are in a tree or on a leopard's fur, are closely associated with the processes of mathematical analysis in Turing's equations. The works by Turing posed two main premises:
- "The repetition of regular patterns in biological systems is regulated by two or more morphogens that work together as activator and inhibitor".16
- "The result of the interaction between activator and inhibitor generates patterns with constants that are determined by the concentrations and diffusion coefficients of the reagents involved".16
One way to understand this would be what Ariel Palazzesi said in 2012, in his article "Alan Turing, animals and their spots":
In vertebrates, skin color is determined by cells loaded with pigments called chromatophores. They have a very particular origin: during formation of the nervous tube, the epithelial cells of the embryonic neural plate margins differ in migratory mesenchymal cells. Some of these cells migrate below the embryonic ectoderm and end up integrated to the epidermis. Depending on their more or less development and accumulation of pigments, they provide the skin with a lighter or darker shade. Ultimately, the way two different chemicals react and distribute on the skin of animals is what provides their hair color. One of them stimulates the production of melanin —the protein that gives the skin its color— and the other blocks its production. Turing's model explains how the different patterns of fur depend only on the thickness and shape of the region where they develop. One group of equations, more or less complex, serves to explain the design of the skin of all animals. In mandarin fish, as the embryo develops the two chemicals are distributed on its surface. The development stage in which these changes take place determine the different designs that are formed. The shape and size of the skin regions impose limitations to the drawings that appear on it. This explains why in an elongated shape —like in a tail— spots are transformed into stripes. These equations also help explain the drawings of butterflies' wings and the colorful tropical fish motifs.17
The confirmation of Turing's theory occurred recently, when the development of regularly spaced crests found in mice palates was studied at London's King College. The study was conducted in embryos, identifying a couple of specific morphogens that work synergistically in the formation of palatal wrinkles: FGF (fibroblast growth factor) and SHH (Sonic Hedgehog). It was found out that when morphogens increase or decrease their activity, they cause a change in the shape of the crests studied, as predicted by Turing's equations.18
Mathematical biology
Mathematical Biology seeks to model biological processes with mathematical techniques.19 The development of this area of knowledge is based on three fundamental premises: the interrelation of biological phenomena, the broad computational development, and the increasing difficulty to vivo experimentation.
A biological process is studied from the mathematical perspective taking into account four steps: delimitation of the phenomenon to be studied, formulation of the assumptions under which the phenomenon is to be analyzed, deduction of the mathematical consequences deriving from the premises, and comparison of the latter with reality.
This means that the consistency of the results obtained in the mathematical analysis is determined by the reliability of the information that contributes to the computational model.
Process of tooth enamel biomineralization
Bioapatite is a biomineral (a crystalline biogenic solid) that forms crystals or solid phases within homogeneous mixtures. Biomineralization occurs in two stages, the first one is called nucleation, forming the first crystal called nucleus, and the second phase consists of growth of the viable nucleus. Of course, for that to happen requires an ionic contribution, so growth will happen only if the solution is supersaturated, meaning it has solute excess to be able to provide the growing crystals with mass.
There are three possible factors that may act individually or as a whole: the temperature that would affect the solubility of the solute, the variability in the amount of solvent, and the third factor is the contribution of a new component that reacts with the solute and forms an insoluble product that will be precipitated when exceeding the Kps.20
From the embryological point of view, teeth are hard structures resulting from the interaction of ectodermal and mesenchymal tissue, which are modulated by signaling pathways, leading to the dental organ differentiation by the expression of the BARX 1 gene, which is also responsible for modifying associated structures such as temporomandibular joint, nearby muscles and salivary glands.21
When the dental organ differentiation starts the third stage called bell stage, the mesenchymal cells of the inner part of the epithelium differentiate to odontoblasts, which begin to produce pre-dentine which mineralizes forming dentin in a gradual process of thickening, which concludes with the odontoblasts in the center of the dental papilla, but leaving behind cytoplasmic extensions that are called odontoblastic (or Tomes') processes. Meanwhile, cells of the inner enamel epithelium differentiate to ameloblasts forming enamel by apposition, retreating to their external epithelium and moving away from the dentin.
Finally, tooth root forms when the inner and outer epithelia of the enamel connect each other at the tooth's neck, giving rise to the sheath of the epithelial root that grows towards the mesenchyme. The internal cells of the dental follicle will form the cementoblasts, which will gradually increase in thickness, resulting in a narrow tube through which the neurovascular package of the formed tooth will pass.22, 23
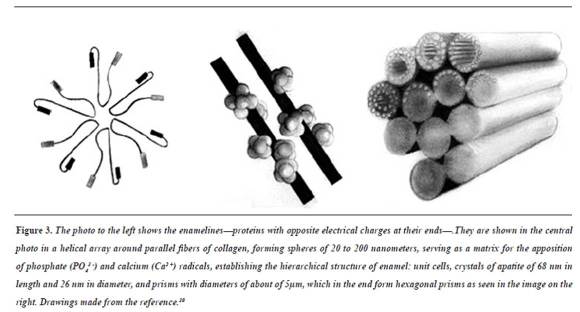
The specific process of tooth enamel biomineralization begins with the ameloblast releasing amelogenins to the extracellular medium;23 these proteins, with positive and negative charges at their ends, join each other in a helical shape, producing a matrix (a spiral staircase) that promotes apposition of compounds with phosphates (PO43-) and calcium (Ca2 +)24 radicals which mineralize by difference of loads25 until forming the hierarchical structure of tooth enamel.26 The sequence of these concepts is schematically shown in figure 3.27-29
DISCUSSION
Parallel between the bases of Liesegang's test and the generation of dental mineral
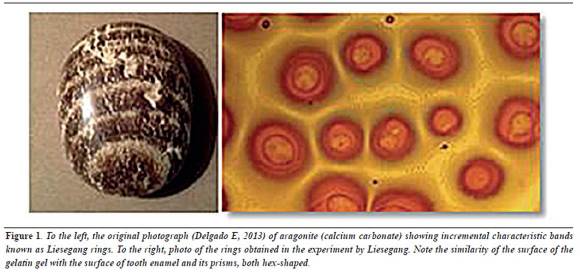
By observing the shape of incremental bands in the original experiment by Liesegang, we see that the growth around each drop is initially circular, but when they meet, the expansive neighboring circles rearrange getting a hex shape (figure 1). Could this be the reason why the enamel prisms are hexagonal?
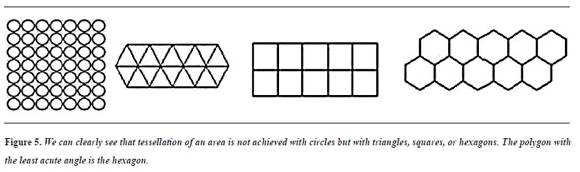
They are two factors that define this situation: tessellation and energy distribution. Tessellation50 is the property of completely covering a flat area with regular polygons,51 without leaving bare spaces or overlying one polygon to another; this is only possible with polygons whose angles are exact submultiples of 360°. There are only three possible solutions to tessellate a plane, namely:
- With equilateral triangles (angles of 60°, which with 6 triangles results in 360° of covered area).
- With squares (angles of 90° x 4 = 360°) and
- With hexagons (angles of 12 ° x 3 = 360°).
Since tooth enamel cannot have empty spaces, nature can select any of these three solutions to configure its structure.
The factor of energy distribution is the second decisive element in enamel configuration; faults in the materials correspond to points or areas of high energy—the more acute the tips the more energy they have and the more they fail, break and dissolve, as they are unstable—. For this reason, for example, free drops are spherical and the planets tend to be round. The circle is the most stable geometric figure, as it has no sharp angles or tips, but by using circles alone one cannot tessellate a plane, as there will always be empty spaces; thus, the final shape of a structure shall be determined by the congruence between complete tessellation and the least acute angle possible.
Of the three regular polygons, the hexagon is the one with the least acute angles and fully tessellates the enamel surface—a solution adopted by nature in other structures such as honeycombs, pineapple shells, and the facets of the eyes of many insects.
CONCLUSIONS
This literature review and the definitions of elements and concepts have enabled to successfully apply these theories to explain and predict the formation of repeating symmetrical patterns in inert materials like rocks of many types and non-biological minerals, as the aragonite in figure 1, as well as in vegetal materials like tree trunks, in biologicallygenerated hard tissues such as the internal spiral chambers of the Nautilus marine snail, which is also of calcium carbonate as the above mentioned aragonite but is generated by cells, in soft tissues like the skin of puffer fish, tigers, and zebras, and every day there are more and more explanations on materials from nature.
In the same way the origin and characteristics of the palatal ridges of mice have been explained, it is expected that in the near future it will be possible to explain in detail the dimensions and minutia of Hunter-Schreger bands, Retzius striae, perikymata, and other symmetric patterns of the enamel which, although in micrographs they look as monotonous as fingerprints, may contain information on the development of each individual and could even serve someday as identification.
The parallel established between rings and enamel highlights several points in common that suggest that the principles of Liesegang, Turing, and Mathematical Biology seem to be applicable to teeth components. To apply these principles to the enamel, it is necessary to be familiar with several enamel properties (viscosity, conductivity, and diffusion coefficients), which have not yet been determined. It is expected that with the current development of measures and instrumentation techniques, this will be achieved in a few years.
CONFLICTS OF INTEREST
The authors declare having no conflict of interest. The study was conducted as part of the coursework of the Master's Research Program at the Universidad Nacional de Colombia School of Dentistry.
REFERENCES
1. Colegio Oficial de Geólogos de España. Diccionario geológico del Ilustre Colegio Oficial de Geólogos de España. 2ª ed. Madrid: CYAN; 1991. [ Links ]
2. Talanquer V, Irazoque G. Phase transitions and universality. Educ Quim 1991; 2(2): 641. [ Links ]
3. Talanquer V, Irazoque G. Auto-organization II: oscillating reactions. Educ Quim 1992; 3(1): 25. [ Links ]
4. Liesegang RE. Ueber einige eigenschaften von gallerten. Naturwissenschaftliche Wochenschrift 1896; 11(30): 353- 362. [ Links ]
5. Stong CL. Las sales reaccionan en un gel para producir bandas de Liesegang en color. Investigación y Ciencia [Internet]. 1977; 8 [Consultado 2013 Sep 14]. Disponible en: http://www.investigacionyciencia.es/investigacion -y -ciencia/numeros/1977/5 [ Links ]
6. Stern KH. The Liesegang phenomenon. Chem Rev 1954; 54(1): 79-99. [ Links ]
7. Boada-Ferrer M. Los anillos de Liesegang. Investigación y Ciencia 2009; 389: 86-88. [ Links ]
8. Sharbaugh III AH, Sharbaugh AH Jr. An experimental study of the Liesegang phenomenon and crystal growth in silica gels. J Chem Educ 1989; 66(7): 589 -594. [ Links ]
9. Boyer W. A textbook of colloid chemistry. 2.a ed. Riverside California: John Wiley and sons; 1956. [ Links ]
10. Ruiza M, Fernández T, Tamaro E, Duran M. Biografías y vidas. 14.ª ed. [Internet] 2004 [Consultado 2013 Sep 2]. Disponible en: http://www.biografiasyvidas.com/ biografia/o/ostwald.htm [ Links ]
11. Glasstone S. Tratado de química física. 7.a ed. Madrid: Aguilar; 1970. [ Links ]
12. Ostwald W. der Lehrbuch der allgemeinen chemie. Leipzig: Engelman; 1897. [ Links ]
13. Valenzuela C. Química general, introducción a la química teórica. Salamanca: Universidad de Salamanca; 1995. [ Links ]
14. O'Connor JJ, Robertson EF. The McTutor history of mathematics archive. [Internet]. [Consultado 2014 ago 13]. Disponible en: http://www-history.mcs.st-and.ac.uk/ Biographies/Turing.html [ Links ]
15. Fernández I, García J, Pacheco J. Bifurcations and Turing instabilities in reaction-difussion systems with timedependent diffusivities. Rev Academ Canaria Ciencias 2004; 1-2: 89-98. [ Links ]
16. Hodges AL. The man behind the machine. Nat 2012; 482: 441. [ Links ]
17. Palazzesi A. Alan Turing. Los animales y sus manchas. [Internet]. [Consultado 2013 Sep 13]. Disponible en: http://www.neoteo.com/alan-turing [ Links ]
18. Economou AD, Ohazama A, Pornaveetus T, Sharpe PT, Kondo S, Basson MA et al. Periodic stripe formation by a Turing mechanism operating at growth zone in the mammalian palate. Nat Genet 2012; 44: 348-351. [ Links ]
19. Miramontes, P. La biología matemática. En: Bautista R. Las matemáticas y su entorno. México: Siglo XXI; 2004. p. 47-65. [ Links ]
20. Perry H. Manual del ingeniero químico. Tomo II. 6.a ed. México D. F.: McGraw Hill; 2000. [ Links ]
21. Thesleff I, Vaahtokari A, Partanen AM. Regulation of organogenesis common molecular mechanisms regulating the development of teeth and other organs. Int J Dev Biol 1995; 39(1): 35-50. [ Links ]
22. Moore KL. Embriología Clínica. 7.a ed. Madrid: Elsevier; 2004. [ Links ]
23. Fincham AG, Moradian-Oldak J, Simmer JP. The structural biology of the developing dental enamel matrix. J Struct Biol 1999; 126(3): 270-299. [ Links ]
24. Munhoz C, Leblond C. Deposition of calcium phosphate into dentin and enamel as shown by radioautography of sections of incisor teeth following injection of 45Ca into rats. Calcif Tissue Res 1974; 15(3): 221-235. [ Links ]
25. Smith CE. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 1998; 9(2): 128-161. [ Links ]
26. Yeau-Ren J, Tsung-Ting L, Hsiu-Ming H, Hsin-Ju C, Dar-Bin S. Human enamel rod presents anisotropic nanotribological properties. J Mech Behav Biomed Mater 2010; 4: 515-522. [ Links ]
27. Wen HB, Fincham AG, Moradian-Oldak J. Progressive accretion of amelogenin molecules during nanospheres assembly revealed by atomic force microscopy. Matrix Biol 2001; 20(5-6): 387-395. [ Links ]
28. Paine ML, White SN, Lou W, Fong H, Sarikaya M, Snead ML. Regulated gene expression dictates enamel structure and tooth function. Matrix Biology 2001; 20: 273-292. [ Links ]
29. Teaford M, Smith M, Ferguson M. Development, function and evolution of teeth. 5.a ed. Cambridge: Cambridge University Press; 2000. [ Links ]
30. Nanci A. Ten Cate's Oral Histology: development, structure, and function. 8.a ed. Philadelphia: Elsevier; 2008. [ Links ]
31. Kato S, Saitoh Y, Iwai K, Miwa N. Hydrogen-rich electrolyzed warm water represses wrinkle formation against UVA ray together with type I collagen production and oxidative -stress diminishment in ?broblasts and cell- injury prevention in keratinocytes. J Photochem Photobiol B 2012; 106: 24-33. [ Links ]
32. Toko K, Nosaka M, Fujiyoshi T, Yamafuji K. Periodic band pattern as a dissipative structure in ion transport systems with cylindrical shape. B Math Biol 1988; 50(3): 255-288. [ Links ]
33. Mahoney P. Intraspeci?c variation in M1 enamel development in modern humans: implications for human evolution. J Hum Evol 2008; 55: 131-147. [ Links ]
34. Guangren Q, Linlin F, Ji-Zhi Z, Yunfeng X, Jianyong L, Jia Z et al. Solubility product (Ksp)-controlled removal of chromate and phosphate. Chem Eng J 2012; 181-182(1): 251-258. [ Links ]
35. Porto I, Merzel J, Barbosa de Sousa F, Bachmann L, Aparecido J, Peres R et al. Enamel mineralization in the absence of maturation stage ameloblasts. Arch Oral Biol 2009; 54: 313-321. [ Links ]
36. Barge LM, Nealson K, Petruska J. Organic in?uences on inorganic patterns of diffusion controlled precipitation in gels. Chem Phys Lett 2010; 493: 340-345. [ Links ]
37. Ngankam P, Schaaf P, Voegel J, Cuisinier F. Heterogeneous nucleation of calcium phosphate salts at a solid/liquid interface examined by scanning angle. J Cryst Growth 1999; 197: 927-938. [ Links ]
38. Dorozhkin SV. Bioceramics of calcium orthophosphates. Biomaterials 2010; 31(7): 1465-1485. [ Links ]
39. Dorozhkin SV. Calcium orthophosphates. J Mater Sci 2007; 42: 1061-1095. [ Links ]
40. Johans C, Kontturi K, Schiffrin D. Nucleation at liquidliquid interfaces: galvanostatic study. J Electroanal Chem 2002; 526(1-2): 29-35. [ Links ]
41. Sasaki T, Debari K, Garant PR. Ameloblast modulation and changes in the Ca, P and S content of developing enamel matrix as revealed by SEM-EDX. J Dent Res 1987; 66(3): 778-783. [ Links ]
42. Hubbard MJ. Calcium transport across the dental enamel epithelium. Crit Rev Oral Biol Med 2000; 11(4): 437-466. [ Links ]
43. Karam T, El-Rassy H, Zaknoun F, Moussa Z, Sultan R. Liesegang banding and multiple precipitate formation in cobalt phosphate systems. Chem Phys Lett 2012; 525- 526(1): 54-59. [ Links ]
44. Chunfang L, Steinar R. SEM observations of Retzius lines and prism cross -striations in human dental enamel after different acid etching regimes. Arch Oral Biol 2004; 49(1): 45-52. [ Links ]
45. Fattibene P, Callens F. EPR dosimetry with tooth enamel: a review. Appl Radiat Isot 2010; 68(11): 2033-2116. [ Links ]
46. Lundgren T, Persson LG, Engström EU, Chabala J, Levi -Settid R, Norén J. A secondary ion mass spectroscopic study of the elemental composition pattern in rat incisor dental enamel during different stages of ameloblast differentiation. Arch Oral Biol 1998; 43(11): 841-848. [ Links ]
47. Yeau-Ren J, Tsung-Ting L, Hsiu-Ming H, Hsin-Ju Ch, Dar-Bin S. Human enamel rod presents anisotropic nanotribological properties. J Mech Behav Biomed Mater 2011; 4(4): 515-522. [ Links ]
48. Habelitz S, Marshall SJ, Marshall GW, Balooch M. Mechanical properties of human dental enamel on the nanometre scale. Arch Oral Biol 2001; 46(2): 173-183. [ Links ]
49. Hong-He L, Swain MV. Understanding the mechanical behavior of human enamel from its structural and compositional characteristics. J Mech Behav Biomed Mater 2008; 1(1): 18-29. [ Links ]
50. Grünbaum B, Shephard GC. Tilings and patterns. San Francisco: W. H. Freeman and Company; 1987. [ Links ]
51. Chavey D. Tilings by regular polygons-II: a catalog of tilings. Comput Math Appl 1989; 17: 147-165. [ Links ]











 text in
text in