INTRODUCTION
Resin-based composite restorations (CRs) have a limited lifespan especially due to the presence of carious lesions in their margins; other causes of failure include fracture of teeth or restorations, marginal damage, dental sensibilities, loss of relationship contacts, and stains or color changes, just to name a few.1)(2)(3)(4)(5)(6)(7)(8
The time a restoration lasts is an important aspect in clinical decision-making. Clinical evidence shows that clinicians spend over 60% of time (ranging from 50% to 75%) in replacing failed restorations.9 In addition, most patients are unaware of their restorations life cycles, and dental services usually lack a comprehensive consideration of restorations lifespan as a budgeting parameter.
Reports on the life expectancy of restorations generally disagree with one other due to factors such as varying study designs, diverse criteria for case selection, different ways to determine success or failure, and survival estimates. Concerning design, these studies rely on prospective and retrospective analysis, and only some of them mention clinicians′ calibration. Prospective studies usually present fewer distortions because they collect data from controlled design studies and consistently observe variables over time; however, these studies require many years to achieve sufficient clinical validation, and there might be bias related to either operator or patient. Retrospective studies are conveniently completed in a short period of time and require lower costs but might imply higher risks of inaccuracies due to omissions. On the other hand, the survival analysis of prospective studies usually presents greater accuracy and the risk of inaccuracies can be counterbalanced by including control cases in which failure time cannot be intervened.6)(10)(11)(12)(13 A number of methodologies have been used to evaluate the quality of restorations but the one adopted by the U.S. Public Health System (USPHS), originally designed by Ryge et al, is the most widely accepted standard nowadays.14)(15)(16)(17)(18
A wide range of factors converge when dentists try to decide whether or not to perform a restoration, ranging from scientific evidence to their personal experiences to patient′s preferences, as well as associated risk factors, costs, and aesthetic factors; particularly important is longevity, as it allows learning about treatment predictability.19)(20)(21)(22)(23 It is well known that longevity is lower in patients at high risk of developing carious lesions because of the presence of secondary caries.1)(4)(7)(12)(24)(25)(26 Some studies show that the highest failure rates are associated with larger restorations and the diverse positions of teeth on the arch, being smaller restorations the ones that enjoy greater longevity,12)(27)(28)(29)(30 while other studies argue that no matter the size of the surfaces to be treated,31 the combination of large restorations and patients with high risk of caries presents high failure rates.32
Variables associated to operators may also affect longevity, as some operators are more efficient than others in achieving high longevity rates. Other variables such as type of restoration and material may also affect longevity; glass ionomer cements are the materials with the lowest longevity. Moreover, patients who change dentists have higher rates of restoration replacements, which affect longevity.2)(4)(27)(33)(34
The average failure rate of CRs is considered to be 2.2% per year,28 being secondary caries and fractures the most common reasons for it;1)(28)(35 replacement has been the traditional treatment but it implies loss of healthy tooth tissue-even in areas far from the failure-and risks of tooth weakening.9)(36 Repairing restorations that have localized defects is an alternative treatment to increase longevity;9)(10)(11)(12)(13)(14)(15)(16)(17)(18)(19)(20)(21)(22)(23)(24)(25)(26)(27)(28)(29)(30)(31)(32)(33)(34)(35)(36)(37)(38)(39 however, these techniques have not been confirmed by Cochrane reviews due to lack of randomized clinical trials.40
Nearly half of all restorations performed by general dentists are replaced because of defects or failures after 10 years.41)(42 The reasons for this replacement are divided into three broad categories:7 factors related to the clinician, properties of materials, and patient-related factors. Regardless of the reasons, it is often difficult to identify which factor was the most determinant for failure. It is sometimes a combination of factors but clinicians rarely record more than one reason for replacement. Most failures occur gradually but they may also occur all of a sudden, as in the case of fractures, where the defect does not necessarily coincide with restoration failure and replacement is immediately indicated. In general, as failures develop gradually, they provide an opportunity to indicate minimally invasive treatments.
During recent years, the literature has included abundant information on treatments to increase longevity through alternative methods such as repairing, sealing, or refurbishing restorations, although this information is scarce in comparison to other methods to overcome technical, chemical, or physical problems in order to enable increased longevity without additional interventions.43)(44)(45
The goal of this literature review is to analyze the findings of different alternatives suggested in the literature to increase longevity of compositeresin restorations and their adhesive bonds.
1. ALTERNATIVE TREATMENTS
1a. CR repair
Repair techniques have appeared in the literature since the 1970s.46)(47)(48 The repair criteria have been established over the past years48)(49)(50 and currently most dental schools include repairing restorations as part of their undergraduate teaching (Japan 95% - USA and Europe 71%).8)(51 The results of published studies deal with repairing marginal damage, secondary caries, or anatomical defects. Gordan et al (after two and seven years) and Martin et al (after four and five years) reported that repairing, sealing, and refurbishing show high survival rates, similar to those of total replacement, improving the quality of restorations with minimally invasive interventions.9)(36)(48)(52)(53)(54)(55)(56
Repair is defined as the removal of part of the restoration adjacent to the defect, as an exploratory cavity, allowing adequate diagnosis and evaluation of extension as well as elimination of the defect; it is then recommended to perform mechanical retentions in the inside of pre-existing restorations, as well as complete isolation with a rubber dam.17
The advantages of repairing have been summarized by Blum et al as the lowest loss and the greatest preservation of healthy tooth structure, reduction of potential damage to dental pulp, pain reduction (usually performed without anesthesia), and less iatrogenic damage to neighboring teeth; in addition, reduction in treatment time and costs, good patient acceptance, and increased longevity of restorations.45
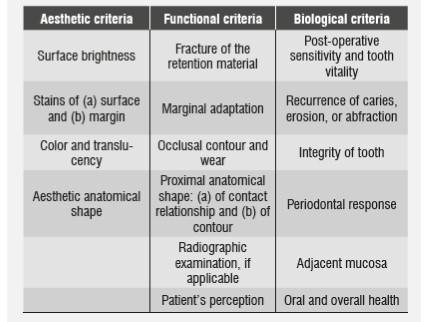
In its 2008 General Assembly, the Scientific Committee of the International Dental Federation (FDI for its French initials) defined the criteria for repair indication, which were confirmed in 2010. These criteria were classified into three groups with subgroups and aesthetic, functional, and biological parameters; each criterion is expressed in five levels: three are acceptable and two are unacceptable (one for repair and one for replacement) (Table 1). The following failures are repairable: defects located in marginal gaps, "splintering" margins, staining margins, fractures of specific portions of the restoration, and secondary caries or wear; size and accessibility should also be considered when repairing.57)(58
In all three groups, the following levels are used for evaluation:
1. Clinically excellent / very good.
2. Clinically good.
3. Clinically sufficient / satisfactory.
4. Clinically unsatisfactory.
5. Clinically poor.
Laboratory studies have provided information on CR repair techniques; for example, in the case of silorane-based composite resins, pre-treatment with sandpaper disks to roughen the surface provides the same results as for methacrylate-based CRs.59 Other recommendations include: using diamond stones,60 sandblasting with aluminum oxide,60 coating with silica,60)(61 and cleansing with phosphoric acid and other bonding agents.59)(60)(61 The use of hydrofluoric acid or silorane primer proved to be less efficient,60)(61 as well as using ozone or cleaning with acetone or ethanol, which did not affect the reparation′s bonding.62)(63)(64 The best results in repairing silorane-based CRs were obtained by combining silane and bonding agent, similar to what happens in BisGMA-based CRs (Bisphenol-a-glycidyl Methacrylate).59)(62)(65)(66)(67 Compatibility of silorane-based CRs and BisGMA-based CRs increase bond strength when silanes and adhesive phosphor-dimethacrylates
are used in the repairing process.59)(60)(68 In general, all aged CR surfaces that were repaired in labs showed reduced traction strength values ranging from 47 to 73% of the cohesive bonding of nonaged resins; in vitro, CRs repaired with nanohybrid fillings show the greatest traction strength values, and the lowest values occur when nanohybrid CRs are repaired with microfilled CRs.(69
One critical topic is defining treatment criteria and selecting patients for repair indication. It is necessary to point out that repair studies in recent years have included localized failures stating that the reminder restoration must be in good conditions; they basically include margin defects, gaps, marginal staining, localized secondary caries and wear, and repair is not indicated in the presence of secondary caries that is inaccessible through a small cavity or when it affects the restoration′s resistance to functional forces because it may compromise deep areas or reduce support for the remaining restoration. Neither is it indicated when the restoration has overall defects, or when the patient rejects alternative treatments or has a history of repair failure; also in cases with two concomitant failures, such as restoration fracture plus marginal defects, in which full replacement is deemed more reasonable.33)(45 Repair indications must be approached flexibly, as clinical history conditions may appear during the repair process, suggesting a change in indication-for example, in the presence of a deeper or more extensive carious lesion than initially observed-. It is also especially recommended to search for a clear cause of restoration failure, whether this variable will affect the repair process, or if it is possible to modify that variable, as in the case of very convex proximal walls with broken restorative material, in which the repair will probably experience the same problem.70 Full replacement remains a necessary indication, since usually not all restorations defects are assessed if they are small, while they often affect a greater extension of the restoration. However, repairs due to caries failures normally have better prognosis compared with restorations failing by fracture.5 Repairing extends the longevity of restorations, reduces the destructive effect of invasive procedures, reduces the probability of switching indications by indirect restorations, and significantly reduces pulp compromise, which is convenient for patients.4)(71
1b. Sealing
Sealing defective margins is another minimally invasive procedure with the ability to greatly increase the longevity of restorations with interventions that are simple, fast, and well tolerated by patients.8)(36)(38)(44)(45)(48)(53)(55)(72)(73)(74)(75 In addition to being a conservative technique, it is rather cost-effective as it requires short times and effectively eliminates marginal defects that could lead to secondary caries.42)(76)(77)(78)(79)(80)(81)(82)(83)(84 Currently there are no publications about its contraindications or recommendations regarding maximum magnitude and the effects of gap location and extension to indicate sealing.
Technically, sealing consists on using sealants or flowable resins52 to eliminate marginal gaps in defective restorations by means of absolute isolation, acid etching, and adhesive procedures, producing clinical results that are similar to those of replacement of restorations after being controlled during five years-the maximum reported period of clinical performance of sealed defective gaps-a time when it should be evaluated whether it is possible to seal the gap again-if the original conditions remain stable-or if it is necessary to apply another restoring technique.37
1c. Refurbishing
Refurbishing implies re-carving anatomic shape defects, eliminating extra contours, and improving surfaces by carving and polishing;39)(82 it has proven to be capable of recovering the morphologic, functional, and aesthetic characteristics of CR restorations with minimal interventions,55)(82)(85)(86 thus increasing longevity. It consists on re-carving occlusal anatomies using carbide or steel burs with multiple blades, discs, and sandpaper bands.55)(86 Limitations of refurbishing include very superficial restorations or risk of permanent damage.
1 d. Reduction of adhesive interface degradation
It is well known that performance and longevity of CR restorations are closely related to quality of the adhesive interface. Preventing or reducing interface degradation has been suggested as a new way to increase the longevity of CR restorations. We′ll analyze studies that show that using active adhesive systems, applying multiple layers of adhesive material, using additional hydrophobic layer, and increasing polymerization times are all techniques that promote resistance to adhesive interface degradation, with clinical consequences such as lower margin staining or prevalence of secondary caries. Furthermore, applying warm air to evaporate the solvent, using a wet technique with ethanol and eventually improving collagen strength through matrix metalloproteinase inhibitors (MMPIs) and collagen protectors, minimize adhesive interface degradation, contributing to increased longevity of CRs.
2. IMPROVING ADHESIVE SURFACE IMPREGNATION
2a. Active application
It has been traditionally considered that the two critical elements to achieve proper resin adhesion to dentin are a) dentin moistening by components of the adhesive material and B9 micro-mechanic assembly through resin penetration in the collagen fibers exposed by acid etching.87)(88
It has been shown that the adhesive agent suffers lower bonding degradation if demineralized dentin is vigorously rubbed for 10 s with two layers of adhesives and manual pressure of about 34 kg/cm,2 followed by evaporation of solvent for 10 s with the tip of the air syringe positioned at a distance of 20 cm plus light curing for 30 s in order to improve impregnation of the active surface, as demonstrated in laboratory tests and clinical results;89)(90 this may happen immediately or in the long term in both dry and wet demineralized dentin. This has prompted debate about generalized techniques in recent years, as dentin must remain hydrated to prevent the collagen mesh from collapsing, avoiding gaps that would block adequate dentin infiltration; in addition, active application provides higher adhesive values. One study shows that the difficulties in keeping dentin moisture and its failures could be overcome by vigorously rubbing the demineralized dentin surface.89
Vigorous application was already known in the case of dentinal adhesives containing the 10 MPD poly-functional monomer (10-metacril-oxo decylphosphate- dihydrogenated), which improves biodegradation resistance of the adhesive interface, resulting in the formation of multiple nano-layers of calcium salts that are attached to the 10 MPD molecule of 3.5 nm thick on each layer of dentin and protect collagen fibers from interphase biodegradation by hydrolysis.90 These nano-layers may explain the high stability of the bonding as well as its physical strength, proven in clinical and laboratory studies,91)(92 thus improving longevity. Interaction with hydroxyapatite occurs with acids of low pH but higher than the traditional ones, so it is necessary to recommend prior selective etching of enamel. The Ca-10-MDP bonding occurs clinically after rubbing the tooth surface for 20 to 30 s.92)(93)(94 This new formulation leaves the traditional considerations of total etching behind and incorporates adhesion to dentin by chemical bonding. As in all new developments, there are still unanswered questions regarding the biochemical secrets of these processes.93)(95)(96)(97)(98
Further studies on the chemical interactions of adhesive interfaces are needed to improve understanding of the activity of Ca-10-MDP nanolayers. The bonding of nano-layers to the dental substrate has been studied and is now understood, but there is not available information on nanolayers bonding in the transition from ultra-adhesive structure to restorative or cementing resin. So far, it has not been explained whether nano-layers are evenly spread over the entire tooth surface or if they are sets of bonding spots in nano-layers; if so, how are the gaps in between nano-layers formed and how they behave, or how they are related to the rest of the hybrid zone?
Theoretically, the size of a molecule of 10-MDP has been established as 1.95 nm; thus, the size of a Ca-10-MDP compound, consisting of two molecules of 10-MDP, would be 3.9 nm.94)(99 It is also known that Ca is 4 nm in diameter. These combined dimensions (7.9 nm) compared to the thickness of each nano-coating of Ca-10-MDP salt (3.5 nm), in both ClearFil SE (CFSE) and Single Bond Universal (SBU), are currently difficult to explain. These dimensional asymmetries could be explained by polymerization shrinkage phenomena in the new Ca-MDP formation; however, this evidence has not been built yet. It has been established that the dimensions of Ca-10-MDP nano-layers are interpreted as a fingerprint that could reveal the functional monomers that make part of the adhesive, but it does not explain the combined dimensions of its components.
It would be especially interesting to have additional knowledge on the interaction with some adhesive components, such as copolymers (from polialkenoic acid) of glass ionomers in adhesives (such as SBU), which could contest for the same HA calcium molecules (hydroxyapatite) used by 10-MDP to form nano-layers. Another important aspect would be to know if the presence of HEMA (hidroetil methacrylate acid) significantly affects the chemical interaction of MDP, preventing the formation of bonds, salts, and nano-layers by reducing the rate of surface demineralization-a pre-requirement for the formation of Ca-MDP salt in its chemical interaction with HA100-and whether such chemical interactions affect the longevity of CRs.
Another original contribution is the recent publication by Mena-Serrano et al, who demonstrated that sonic application of the adhesive to a 170 Hz oscillation frequency can improve the µTBS of dentin/resin bonding, reduce nano-leakage, and slow down the degradation of adhesive bonding.101
2b. Application of multi-layer
Laboratory studies have shown that application of multi-layer adhesives can increase adhesion in the presence of leakage by increasing adhesives values. A clinically-controlled, randomized, prospective study on the behavior of CR restorations made with self-etching and etch-and-rinse adhesives in non-carious cervical lesions (Loguercio and Reis) concluded that the multi-layer adhesive technique significantly improves retention rates in both adhesive techniques 12 and 18 months after application; however, it does not reach the level required by the ADA (American Dental Association′s Guideline) for bonding between dentin and adhesive materials. It is worth noting that the greater the amount of adhesive layers the lower the amount of failures in Class V adhesive restorations,102 making the application of a double layer of adhesive a clinical standard-but accompanied by just one light-curing process-. This is an original contribution easy to follow, presented by only one publication, and therefore more reports should be expected before considering its incorporation in daily clinical use.
2c. Application of an additional hydrophobic layer
The clinical performance of adhesives has significantly improved in recent times, achieving current adhesive restorations with higher levels of predictability in terms of clinical success. Modern adhesive systems are better than the early ones in terms of retention.103)(104 However, the chemistry of self-etching adhesive systems remains a great challenge, as it incorporates hydrophilic and hydrophobic monomers, resulting in highly hydrophilic systems, which produce semi-permeable membranes with water diffusion from dentin through the adhesive layer105-a process that decreases the mechanical properties of polymers in the resinous matrix-.106 Retention of residual water because of incomplete evaporation of the adhesive agent or due to moisture from underlying dentin as a result of high osmolarity of adhesives produces channels filled with water inside the adhesive material. (105 As a contribution to the solution to this problem, it has been suggested incorporating a hydrophobic layer on the self-etching adhesive. Experimental and clinical tests107)(108 have demonstrated that including an extra hydrophobic layer in addition to the self-etching adhesive procedure improves the clinical behavior observed 6, 12, and 24 months after application, mainly in terms of retention of CR restorations performed on Class V non-carious cervical lesions, as a result of lower degradation of adhesive bond.90)(109)(110 However, longer-term clinical studies are required to complement this information.
Recently, observations by Muñoz et al suggested that reducing nano-leaking by applying an additional hydrophobic layer is dependent on the composition of the adhesive agent rather than on the adhesive strategy itself.111
2d. Late polymerization or passive evaporation of solvent
Dentin adhesives contain monomers of hydrophilic resins, which are dissolved in organic solvents such as acetone and ethanol. Using these solvents favors the movement of water from dentin surface and facilitates monomer penetration to the microporosities exposed by acid etching.112)(113 Including a solvent has increased adhesive bond values and its presence along with water is considered essential for the plastic behavior of collagen bundles and for managing their collapsing.114)(115 Ideally, solvents must be completely removed before light-curing the adhesive in order to prevent unwanted effects to monomers,116 such as reduction of their mechanical properties, poor polymerization, formation of cracks on adhesive agent, and premature failure.117)(118)(119)(120
A lower degradation of adhesive bonding has been observed when slowing down the polymerization of adhesive monomers; in addition, under this conditions, the micro-traction strength values increase if waiting 300 s before light-curing.121 As early as 2002, one published study on the Scotch-Bond adhesive system claimed that increasing the waiting time to 30 s before light-curing significantly increased shear strength.121 The explanation to this phenomenon may be related to the fact of giving the adhesive agent more time to evaporate its alcohol solvents and water, especially since it has been well known that adding water to adhesive systems promotes the formation of water channels through the hybrid zone, when it is associated to alcohols; this happens because adding water to both comonomers and ethanol increases the retention of both ethanol and water in the system, because the two solvents can join the hydrogens of monomers.122 Increasing exposure time for evaporation of the adhesive system after its application to even more time than that recommended by manufacturers does not prevent its degradation but can increase bond strength immediately and for up to six months, perhaps as a result of the amount of polymer chains;110 unfortunately, the literature lacks additional studies on higher times.
3. IMPROVEMENTS TO POLYMERS STRENGTH
3a. Using hot air to evaporate solvents
Initially, water absorption in resins was considered favorable as it compensated the effect of shrinkage by polymerization;123)(124 however, water absorption is currently associated with internal resin weaknesses, which facilitate the extraction of free monomers or residual materials from the polymerization of CR. Water molecules can form clusters that induce softening and deformation of the matrix that surrounds it, decreasing the restoration rigidity.125)(126
Degradation and the shorter longevity of RC restorations are related to deleterious phenomena at the hybrid zone, with degradation of collagen fibers inadequately protected.105)(127)(128 Several clinical approaches have been developed in search for solutions to this conflict, one of them being the incorporation of a flow of hot pressurized air to promote forced evaporation of the adhesive agent′s solvents, concluding that this method can improve the bonding interface over time (six months), especially in water/ethanol-based adhesive systems.129)(130 This is based on the improved quality of the hybrid layer considering its ability to produce lower nano-leakage and to reduce the number of pores, apparently without modifying the resins′ conversion degree.131 A recent study shows that using hot air to evaporate solvents was not efficient in reducing water absorption or adhesive agents solubility, keeping the increase in adhesive bond values.127 The truth is that evaporation of solvents remains and unsolved issue, since the evaporation times recommended by manufacturers for acetoneor ethanol-based adhesive agents retain 5% to 10% after having been blown for 120 s122-a period more than 10 times higher than recommended-, but when the solvent is mixed with 30% of water it can retain up to 41%.122
From the perspective of pulp biology, it is well known that several factors affect the temperature increase in pulp (for instance, in terms of residual dentin thickness), as dentin has a low thermal conductivity and acts as a shield against thermal action;132 however, there are no studies showing the safety of this approach, which is at least deemed unfavorable by histological studies on vital units; studies on endodontically-treated teeth are then suggested
3b. The ethanol-wet technique
The studies by Sadek et al show that another antidegradation strategy for adhesive bonding is the application of ethanol on dentin surface prior to the adhesive technique. The suggested mechanism of action is saturation of surface by ethanol and water movement, preventing integrity of the hybrid zone by absence of water, according to laboratory observations after 9 and 18 months along with TEM analysis (Transmission Electron Microscopy), in a study conducted with reproduction of dentin hydraulic pressure.133)(134 However, despite showing no degradation of the hybrid layer, from the clinical point of view it will be difficult to implement for the time being, given the undemonstrated biological effects of ethanol application on dentin pulp.
4. IMPROVING THE STRENGTH OF COLLAGEN FIBERS
4a. MMP inhibitors
It is well known that the dentin matrix contains matrix metalloproteinases (MMPs)135)(136)(137 and that some of these MMPs attack collagen (MMP 8 and 20) and gelatin (MMP 2 and 9).136)(138 Dentin contains MMP 2 and 20)(138)(139 and the activity of MMPs derived from host enzymes degrade the hybrid layer in vivo.140)(141
Pretreating dentin with 0.2% chlorhexidine (CHX) for 60 s prevents the collagenolytic activity to levels close to zero142 through inhibition of the activity of MMPs in the hybrid layer, increasing its longevity; unfortunately these studies have been conducted in vivo for the maximum period of 14 months,143 showing that it does not decrease adhesive values in Class V restorations. Besides CHX, other compounds such as EDTA (ethylenediaminetetraacetic acid) produce an inhibitory action on MMPs in collagen degradation in demineralized dentins. MMPs degradation was reduced in resin-infiltrated dentin, and the presence of another element such as zinc produced an additional protective effect.144
Pashley et al have named these new compounds "therapeutic adhesive systems" because they have antibacterial and anti-MMP activity as they contain CHX and benzalkonium chloride.145
Massoni et al claim that using non-toxic MMP inhibitors such as CHX is an additional step in adhesive systems in order to increase the longevity of adhesive restorations.146
Although this scheme has detractors, recently Luhrs et al conducted laboratory analysis noting that the action of MMP inhibitors fails to prevent the decreased micro-traction strength after their samples had been aged; in addition, it does not improve longevity when used in cementing composite resins.147
These results are promising but in vivo randomized trials are needed in the long term in order to assess the exact dimension of these investigations.
4b. Collagen protectors - proanthocyanidin use
It is known that durability of resin-dentin bond requires stable collagen fibers in the hybrid layer; however, using simplified adhesives to demineralize dentin activates the endogenous matrix metalloproteinases, resulting in progressive loss of unprotected collagen fibers.146)(148 Conversely, involvement of endogenous MMPs in the degradation process is minimal, when the self-etching adhesives are softer.90)(149)(150 Modifying the demineralized collagen matrix with external crosslinking agents plays an important role in improving the biomechanical properties of dentin.151 Agents of collagen fibers reinforced by crosslinking show that it decreases the enzymatic degradation, and are therefore considered critical to increase the hybrid layer stability as well as durability of the restoration adhesion. Examples of agents often used as external crosslinkers in dentistry are glutaraldehyde, genipin, carbodiimide, and proanthocyanidin (PA).
PA is a polyphenolic compound classified as a flavonoid plant, which is part of the tannins group; it is found in the bark of pine trees and elm trees, and in grape seeds;1)(52 it is also available in vegetables and fruits but in lower concentrations. PA is a powerful antioxidant and a crosslinking agent of low toxicity. It has been shown that PA from grape seeds extracts improve the traction strength and the rigidity of dentin collagen,151)(153)(154 as well as the long-term stability of dentin collagen matrix.155)(156 In addition to its crosslinking effect, the PA obtained from elm and blueberry extracts has also shown the ability to inhibit the production of MMPs 1, 3, 7, 8, 9, and 13 in macrophages, as well as the catalytic activity of MMPs 1 and 9.157)(158)(159)(160
Dos Santos et al reported that experimentally pretreating demineralized dentin with PA for one hour before applying the adhesive agent significantly improves the nano-mechanical properties (nanohardness and modulus of elasticity) of the resin/dentin interface,158)(159 as well as bond strength.161 Similar results have been observed in studies on dentine affected by carious lesions;162 however, because of the time of application, it is impossible to use this protocol in clinical conditions, and that is why the inclusion of PA in the dental adhesive has been considered, as it allows the PA to act for a long period of time, increasing the crosslinking degree with collagen and resistance to biodegradation.163 Adding PA in an adhesion experiment showed that it had no adverse effects on adhesive bond strength when used in concentrations of up to 2% besides significantly reducing nano-leakage.150 Other observations suggest that using ethanol as adhesive solvent promotes PA/collagen interaction by decreasing the dielectric constant of the adhesive agent and enhances the stability of hydrogen bonding.164 The mechanism of action of PA preserves the triple helix collagen structure and induces the addition of microfibers by displacing water and creating a new hydrogen/ collagen bond.165 Promising experimental results may become available for clinical use in the coming years.
Recently a group of researchers has drawn attention by observing that crosslinking agents are effective in reducing MMPs activity by mixing 0.5% carbodiimide (EDC) and 35% HEMA without affecting the roles of each component.166
CONCLUSIONS
Using minimally invasive techniques such as repair, sealing, and refurbishing defective composite resin restorations show longitudinal clinical evidence in randomized clinical trials which increase longevity in restored teeth.
In addition, other innovative methodologies with clinical evidence still in progress, such as improving impregnation of adhesive surfaces, increasing the strength of adhesive polymers and increasing the strength to collagen hydrolytic degradation, are promising steps that either together or separately modify the management of adhesive techniques and will offer alternative restorative treatments to the population demanding aesthetic solutions of composite resins with longer longevity.











 text in
text in