INTRODUCTION
Amelogenesis
The morphogenesis of dental organs begins during the sixth week of intrauterine life in humans (approximately at forty-five days), and in mice it begins during the E12.5 stage.1 The first indication is the differentiation of dental lamina or slat tooth, originated from the ectoderm that covers the oral cavity. The ectomesenchyme induces oral epithelial basal cells to proliferate and form two new structures: the vestibular lamina and the dental lamina. The vestibular lamina forms the vestibular groove while the dental lamina generates the corresponding epithelial growth of future teeth: 20 deciduous teeth and 32 germs of permanent teeth. According to their morphology, dental germs will continue their evolution in these stages: stage of massive outbreak (or yolk), cap stage, bell stage, and stage of tooth follicle, either terminal or mature.
It is at the bell stage the enamel organ presents a new layer-the intermediate layer-located between the inner epithelium and the stellate reticulum. Histogenesis or apposition of the dental hard tissues (enamel and dentin) begins at the end of the bell stage. Cells forming the inner epithelium, also called pre-ameloblasts, differentiate from young ameloblasts and will be responsible for enamel production.
Enamel, also known as adamantine substance or tissue, is located in the coronal portion covering the underlying dentin. It originates from the enamel organ, and is the hardest tissue of the body. It consists of enamel prisms, which are highly mineralized, located from the dentin-enamel junction (DEJ) to the external surface in contact with the oral cavity. Hydroxyapatite crystals form the inorganic enamel matrix and represents 95% of it. These are composed of calcium phosphate and are densely compacted. The organic matrix of enamel is protein in nature; however, collagen is not involved in its chemical composition, having a reduced proportion representing 0.36-2%.
The bell stage will be of great importance for the formation of the coronal morphology by the action of specific signs of adjacent ectomesenchyme on this inner epithelium. This coronal pattern will be determined prior apposition and mineralization of dental tissues.
Ameloblasts are cells responsible for the secretion of organic matrix, and during the formation of the tooth germ they go through a series of steps or stages, which are characterized by functional and ultrastructural changes dependent on cell activity, according to the formation processes or enamel maturation. This is why there are four important stages in the development of enamel: pre-secretory, secretory, transition, and maturation.2
Enamel development or amelogenesis includes two stages: a) the formation of extracellular organic matrix and b) the process of matrix mineralization, which at the same time includes the formation and elongation of crystals and the elimination of organic matrix and crystals maturation.
Ameloblasts differentiate from the inner epithelium of the enamel organ. For this process to take place, it requires the presence of dentin. This stage is called pre-secretory, which prior to the formation of mineral requires pre-dentine deposition by odontoblasts in the future dentin-enamel junction.
During the secretory stage, pre-ameloblasts are transformed into secretory differentiated cells, which are highly specialized and have lost the ability to undergo mitosis. They are columnar cells of nearly 60 µm in height. At the ultrastructural level, there are abundant mitochondria, Golgi complex, and RER distributed throughout the entire cell and more developed in the proximal pole. It presents microfilaments of tubulin, Α-actinin, vinculin, and pre-keratins. In addition, it has vesicles called ameloblastic bodies or enamel bodies, which are granular formations and are precursors of the organic matrix of the enamel. Their content is not known with accuracy, but they are believed to be protein in nature and some authors consider that they can have mineral calcium salts in soluble form.
Once these ameloblastic bodies are formed in the Golgi complex, they migrate to the proximal pole of ameloblast, where they are released towards the formed dentin. This is how the first layer of aprismatic enamel forms. Ameloblasts move away from the surface of the dentin forming the Tomes processes-a structure responsible for the formation of crystals-. While this happens, the ameloblast produces four different proteins and secretes them into the enamel matrix. Three of these are structural proteins and one is a proteinase. Amelogenin (AMELX) represents 80- 90% of the organic matrix, while ameloblastin (AMBN) and enamelin (ENAM) represent 5 and 3-5% respectively. Proteinase, which is a matrix metalloproteinase 20 (MMP-20, enamelysin) is in variable amounts. The total thickness of the enamel layer is achieved at the end of the secretory stage.
The beginning of the transitional stage varies among species and according to the developing tooth. At this stage, the ameloblast reduces in size, while it′s transverse diameter and Golgi complex increase and its RER decreases in volume. The Tomes′ process disappears and in the proximal pole there are microvilli and tubular invaginations. These structures allow them to have absorptive capacities and to eliminate water and organic matrix from the enamel. This facilitates the subsequent increase of organic component and the transformation to mature enamel. At this stage, ameloblasts synthesize calcium-dependent ATPase as well as lysosomic enzymes and alkaline phosphatase. 25% of ameloblasts die in the transition phase, and another 25% dies in the maturation stage.
When the mature enamel has formed, the ameloblast enters in a state of regression, and the cells merge with the rest of the layers of the enamel organ. These strata form a stratified layer known as reduced enamel epithelium, functioning as protection of mature enamel. If this epithelium degenerates prematurely, there can be no tooth eruption.3
Matrix metalloproteinase 20 (MMP-20) / Enamelysin
Matrix metalloproteinases (MMPs) are a family of proteinases secreted as proenzymes or inactive zymogen responsible for the degradation of extracellular matrix (ECM) components, and their activity is regulated by its endogenous inhibitors or TIMPs. MMPs are involved in normal processes of embryonic development, in reproduction (endometrial cycle) and maintenance (bone remodeling), as well as in pathological processes such as the destruction of tissue (periodontal disease, tooth decay, pulp inflammation) and fibrotic diseases (multiple sclerosis). Members of this family have been classified into six groups, according to their structural and functional organization.4
The group of collagenases belong to the MMP-1, MMP-8, MMP-13, and MMP-18. These enzymes are responsible for degrading interstitial collagen I, II, and III and other components of the extracellular matrix. The next group is the gelatinases: gelatinase A (MMP-2) and gelatinase B (MMP-9). These are responsible for the degradation of denatured collagen and laminin. The third group are the stromelysins: stromelysin-1 (MMP-3) and stromelysin 2 (MMP-10), which degrade various components of the MEC. These include fibronectin, laminin, and proteoglycans and non-fibrillar collagens IV, V, IX, and X. Matrilysins are characterized by the absence of hemopexin domain, and include the matrilysin 1 (MMP-7) and matrilysin 2 (MMP-26). Membranetype Metalloproteinases (MT-MMPs) are: MMP-14, MMP-15, MMP-16, MMP-17, MMP-24, and MMP-25. Finally, there are seven MMPs that have not been classified in any of the abovementioned groups, including enamelysin (MMP-20) which is responsible for amelogenin degradation.5
In recent years, it has been shown that the protein component of mature enamel significantly decreases in comparison with immature enamel. During amelogenesis, there exist the following components of the organic matrix of enamel: tuftelin, also known as fringe protein, and dental sialophosphoprotein (DSP) in the amelodentinal junction. Amelogenins appear later, making up 90% of the organic matter and decreasing as the enamel progresses to its maturation stage. Enamelin and ameloblastin form in the last place, with participation of the MMPs that are present in the secretory phase of ameloblasts and serine proteases, which are associated in the superficial part of enamel crystals and the maturation stage.
Several studies suggest that, in developing enamel, MMPs participate in a change of the components of the organic matrix when ameloblasts move from the secretory phase to the maturation phase. It has been shown that MMP-20 has the ability to split amelogenin and enamelin and is the only MMP during the secretory phase. It is also capable of splitting the pro-KLK4 (4 kallikrein-related peptidase) to produce active KLK4 serinprotease. It has also been shown that the MMP-20 degrades E-cadherin, casein and/ or gelatin, agrecane, type IV and type V collagen, tenascin-C, laminin-1, and laminin-5.6
Enamelysin or MMP-20 was originally cloned from porcine enamel organ, and by in situ hybridization techniques it has been shown that both ameloblasts and odontoblasts present transcripts of MMP-20.7 Also, very few cell lines express MMP-20, such as tooth development and the large intestine, but its expression has not been found in tissues such as small intestine, kidney, liver, pancreas, brain, lung, spleen, or stomach.8 This MMP was found in some pathological conditions such as calcifying odontogenic cysts, odontogenic tumors, and cells of human tongue carcinoma.9)(10)(11 For these reasons, it is considered an MMP specific to tooth.
The importance of enamelysin in tooth development has been highlighted in recent decades, specifically in the formation of enamel, and in how alterations in their expression can lead to the formation of pathologies such as amelogenesis imperfecta.12)(13 For this reason, the objective of this review is to classify the different studies and laboratory techniques, showing the participation of enamelysin in tooth development and its relation to pathologies in the formation of enamel.
METHODS
A systematic review was conducted with the following bibliographic databases: PubMed, Science-Direct, Hinari, and SciELO, using these keywords: enamel, tooth development, enamelysin, MMP- 20, amelogenesis imperfecta, in order to classify the different studies related to the participation of MMP-20 in tooth development and the methods used to detect its expression, between the years of 2009 and 2014.
RESULTS
This systematic review presents the different studies in in vitro models, cell cultures, and animal studies as well as in humans, related to enamelysin and tooth development. 19 references were taken into account. The results are listed in Table 1.
Table 1 Studies published in the literature on the different methods used to detect the expression of MMP-20 in tooth development
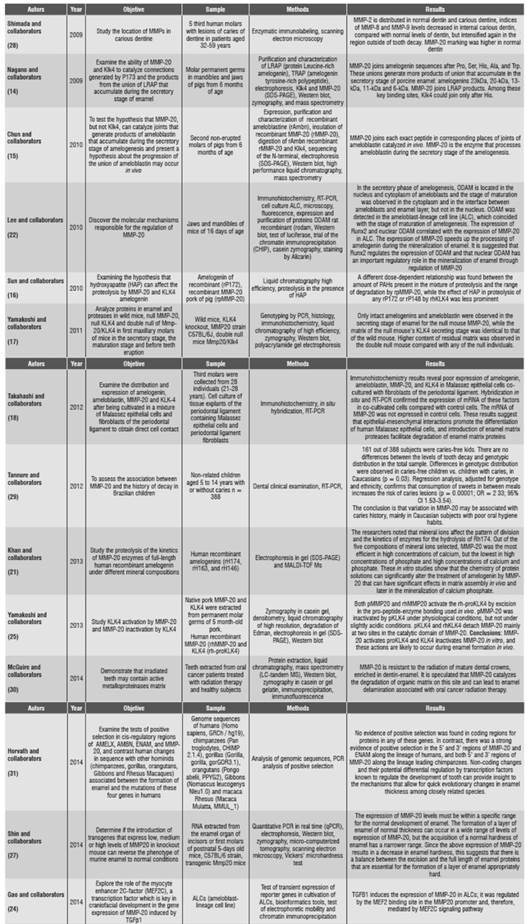
Abbreviations: SDS-PAGE (polyacrylamide gel electrophoresis), ALCs (ameloblast-lineage cell line), MALDI-TOF (matrix-assisted laser desorption/ionization), ODAM (odontogenic-associated ameloblasts protein), RT-PCR: reverse transcriptase polymerase chain reaction.
DISCUSSION
In vitro models
In 2009, Nagano et al.,14 in an in vitro model showed, through the digestion of fluorescent peptides, that MMP-20 is able to digest amelogenin sequences in specific sites. On the other hand, Klk4 (kallikreinrelated peptidase 4) has affinity for different excision sites. Therefore, in the development of pig tooth, MMP-20 is the only that presents proteolytic activity in the extracellular space during the secretory phase. This is confirmed by Chun et al.,15 who concluded that MMP-20 -and not Klk4- catalyzes enamel proteins that are processed during the amelogenesis secretory phase. Other authors, such as Sun et al.,16 studied MMP-20 and Klk4. They designed a series of in vitro experiments systematically assessing the effect of HAP (hydroxyapatite) in rP172 (recombinant porcine amelogenin), rPMMP-20 (recombinant pork metalloproteinase 20), and rP148 (recombinant porcine amelogenin lacking 24 hydrophilic C-terminus amino acids) by rhKLK4 (recombinant human kallikrein-related peptidase 4). They found out that the activity of rPMMP-20 against the recombinant porcine amelogenin was more sensitive to the presence of hydroxyapatite crystals compared with the rhKLK4 against rP148, or rhKLK4 against rP172.
This suggests that there is an intimate relationship between the step-by-step degradation of amelogenin in the presence of HAPs in the early stage of enamel mineralization. Similarly, in 2013, Yamakoshi et al17 concluded that MMP-20 activates proKLK4, and KLK4 inactivates MMP-20 in vitro, and these actions can happen in vivo during amelogenesis.
These findings disagree with those by Takahashi et al18 in 2012. These authors designed an in vitro model with cell cultures of tissue explants from periodontal ligament, containing Malassez epithelial cells and fibroblasts of the periodontal ligament of lower third molars of 28 individuals aged 21 to 28 years, in order to determine the expression of amelogenin, MMP-20, (KLK4) and their effects on the interaction between Malassez epithelial cells and fibroblasts of the periodontal ligament. The immunohistochemistry results showed a weak expression of amelogenin, ameloblastin, MMP-20, and KLK4 in Malassez epithelial cells. This may happen because the functions of these proteins in enamel and their proteases in the formation of dental roots are still unknown.
On the other hand, Bromley et al19 studied the growth of calcite crystals in the presence of full-length amelogenin and its proteolysis by a recombinant matrix metalloproteinase (rhMMP-20). Recombinant porcine amelogenin (rP172) alters the shape of calcite crystals by inhibiting the growth of the steps in the sides that are occluded in the crystals. These authors found out that, in samples with rP172-rhMMP-20, occlusion of the amelogenin in calcite crystals decreased drastically. The c-terminal end reduction decreased the affinity of the amelogenin crystals and therefore foresaw the occlusion, as found in studies by Uskokovic et al.,20 who by means of an in vitro model determined the ability of amelogenin to promote the nucleation and growth of crystals.
Concerning the process of mineralization and formation of mature enamel, Khan et al21 studied proteolysis of the kinetics of MMP-20 enzymes of full-length human recombinant amelogenin under different mineral compositions. They found out that MMP-20 was more efficient in high concentrations of calcium, but lower in high concentrations of phosphate and high concentrations of calcium and phosphate. These in vitro studies show that the chemistry of protein solutions can significantly alter the treatment of amelogenin by MMP-20, which can have significant effects in the matrix assembly in vivo and later in the mineralization of calcium phosphate.
Cell cultures
In 2010, Lee et al,22 studied the molecular mechanisms responsible for the regulation of MMP-20, finding out that the secretory phase of amelogenesis ODAM (odontogenic- ameloblast-associated protein) was located in the nucleus and cytoplasm of ameloblasts, and maturation stage was observed in the cytoplasm and at the interface between the ameloblasts and enamel coating, but not in the nucleus. It is suggested that Runx2 regulates the expression of nuclear ODAM playing an important regulatory role in the mineralization of enamel through regulation of MMP-20. These results were verified in 2012 by Lee et al.23 These authors evaluated the expression of ODAM in ameloblasts, odontoblasts, and several types of cancer cells, finding out that ODAM was located in these cells both in vivo and in vitro. These results suggest that the pattern of expression and subcellular location of ODAM is highly variable and dependent on cell types, their stages of differentiation, and the functional correlations between ODAM and MMP-20.
On the other hand, Gao et al24 explored the role of the myocyte-specific enhancer factor 2C (MEF2C) and transforming growth factor beta 1 (TGFΒ1) in the gene expression of MMP-20. These authors concluded that the TGFΒ1 induces the expression of MMP-20 in ALCs (ameloblast-lineage cell line).
Studies in animals
Concerning studies in mouse models, Yamakoshi et al25 characterized knockout mice for MMP-20, KLK4, and double null MMP-20/KLKL-4. They studied the secretory and maturation phases by histology, zymography, and Western blot. They only found intact amelogenins and ameloblastins in the secretory phase in knockout mouse for MMP- 20, while the matrix belonging to the secretory phase for the null mouse KLK-4 was identical to that found in wild mouse.
More matrix remnants were observed in double null mice compared with knockout mice of MMP- 20 or KLK-4. This was supported by Bartlett et al26 and Shin et al,27 who by means of a knockout mouse model for MMP-20 showed the importance of MMP-20 as a necessary mediator for the maintenance of enamel surface, a strong dentinenamel junction, and the establishment of enamel rods pattern. These results validate the findings in in vitro models by Nagano et al14 and Chun et al.15
Studies in humans
With regard to studies in humans, they mention the importance of MMP-20 in dental caries, enamel thickness, and susceptibility to caries by radiation and dental agenesis.
By means of immunolabeling enzyme, Shimada et al28 studied the participation of MMP-2, -8, -9, and -20 in dentinal caries lesions in third molars of human patients aged 32 to 59 years, finding out that the MMP-2 was distributed in normal dentin and carious dentin, MMP-8 and MMP-9 levels were significantly decreased in inner dentinal caries compared to normal dentin, and MMP-20 was the highest in normal dentin, significantly decreasing towards the outer region of caries.
On the other hand, Tannure et al29 evaluated the association of MMP-20 and caries history in Brazilian children. 161 out of 388 subjects were caries-free kids. The authors found no differences between the levels of tooth decay and genotypic distribution in the total sample. However, there were differences in genotypic distribution. Differences in genotypic distribution were observed in caries-free children vs. children with caries, in Caucasians (p = 0.03). These results suggest that caries development is multifactorial and may be associated to MMP-20 genotypes, in higher proportions among Caucasian individuals with poor oral hygiene habits.
In 2014, McGuire et al,30 by means of mass spectrometry techniques, immunolabeling, and gel zymography, studied the teeth of patients with oral cancer treated with radiotherapy, comparing them against the teeth of healthy individuals. They concluded that MMP-20 is the most abundant matrix metalloproteinase in irradiated and incubated in vitro extracts of mature dental crowns, as in control crowns. In their in vitro studies, they found out that the 23 kDa fragment of MMP-20 is resistant to radiation of mature dental crowns, which indicates that this component can degrade the protein components of the enamel-dentin interface and thus contribute to the pathological delamination observed in oral radiotherapy, which is also associated with high doses of radiotherapy.
In the same year, Horvath et al31 found strong evidence for a positive selection in the 5′ and 3′ regions of MMP-20 and ENAM regions along the lineage of humans, and both 5′ and 3′ regions of MMP-20 along the lineage leading chimpanzees. Non-coding changes and their potential differential regulation by known transcription factors regulate tooth development and can provide insight into the mechanisms that allow quick evolutionary changes in enamel thickness among closely related species.
On the other hand, Kuchler et al32 studied the associations of MMP-1, MMP-3 and MMP-20, and their relations to dental agenesis in 167 nuclear families of two different populations, 116 from Brazil and 51 from Turkey. The participants had at least one missing tooth. These authors found associations among dental agenesis, MMP-1 (p = 0.007) and MMP-20 (p = 0.03) in Brazilian families. These results disagree with those reported by Antunes et al,33 who studied the association of polymorphisms in human genes for MMP-2, MMP-9 and MMP-13 with dental agenesis, in 285 non-related individuals (202 control individuals without dental agenesis and 83 cases with dental agenesis), finding out no significant association for the MMP-2 genotype, but a significant relationship of dental agenesis associated to MMP-9 and MMP-13 genotypes.
CONCLUSIONS
The results of this review highlight the importance of MMP-20 in the degradation of various proteins of organic matrix, such as amelogenins and ameloblastins in the secretory phase of enamel formation, in addition to the involvement of other proteases such as KLK4 which, unlike MMP-20, takes part in the maturation stage of amelogenesis. The in vitro models allow concluding that MMP-20 has specific sites for excision of matrix proteins and that these differ to KLK4. Similarly, this process may be altered by chemical composition, ions, and the presence of hydroxyapatite.
The knockout models allow concluding that alterations in MMP-20 completely alter enamel morphology, unlike the findings in null mice for KLK4. These models allow us to understand the different variations occurring during enamel development, such as amelogenesis imperfecta.
In human studies, MMP-20 has been related to a) increased susceptibility to tooth decay, b) an evolutionary relationship between humans and chimpanzees, as well as an association with enamel thickness, and c) a greater presence of MMP-20 in the amelodentinal junction in teeth subjected to radiation therapy, which is linked to enamel delamination. Finally, there is an association between MMP-20 and dental agenesis.











 text in
text in 


