INTRODUCTION
Hemifacial microsomia (HFM) corresponds to a spectrum of congenital craniofacial malformations characterized by hypoplasia of tissues embryologically originating from the first and second branchial arches. Its expression is highly variable, even with defects of heart, spine, and central nervous system.
It is the second most common craniofacial malformation after cleft lip and palate, with an estimated incidence of 1/5,600 births.1) It appears one-sided in 70% of cases, and its bilateral form is usually asymmetrical, affecting one side more than the other.2
As a complex heterogeneous alteration, patients showing its wide manifestations have received various diagnoses, such as Goldenhar Syndrome, oculo-auriculo-vertebral spectrum, syndrome of the first and second branchial arches, craniofacial microsomia, and among others. There are no common diagnostic criteria for this disorder yet.3
Its pathogenesis responds to a heterogeneous character explained by different theories.4) One of them has been suggested by Poswillo, who claims that the cause would be a vascular disruption causing bleeding during embryologic formation of the stapedial artery, accompanied with alterations in the development of the first and the second branchial arches. The size of hematoma and the resulting tissue injury would explain the morphology and different variations of HFM in experimental models, since the larger in size the larger the alterations in the development of branchial arches.5) Another theory has been postulated by Johnston,6) who claims that the causal factor would be an alteration in the migration of cells of the neural crest towards the formation of the trigeminal ganglion. This lack in migration, and therefore the absence of interaction between mesenchyme and neural crest cells, has been associated with other problems observed in HFM patients, such as microdontia and hypodontia,7) cleft palate and heart problems.8) Other authors suggest this relationship between lack of migration of cells of the neural crest and HFM because in the absence of these cells there is less vascular endothelial growth factor (VEGF). This growth factor promotes the proliferation of Meckel cartilage, and the absence of VEGF creates a correlation with mandibular hypoplasia.9
While its etiology is still unknown, various environmental and genetic factors have been recognized. Within the environmental causes, there are various risk factors associated with its presence during pregnancy, such as vasoactive medications,10) vaginal bleeding during the second quarter, multiple gestations,11) use of assisted reproductive technology by the mother12) and preexisting or gestational diabetes.13)(14
M. Werler has published several studies on the relationship between HFM and the consumption of vasoactive drugs by the mother during pregnancy. One of them is a retrospective study of cases and controls discussing several factors to which the mother is exposed to during pregnancy, including vasoactive drugs such as pseudoephedrine, phenylpropanolamine, aspirin, and ibuprofen. The use of vasoactive medication in the first quarter, particularly in combination with smoking, was found to be associated with an increase in the risk of HFM; other factors were also associated, such as multiple gestations, diabetes, bleeding during the second quarter, and intensive alcohol consumption.15
Within the genetic causes, even though most cases are sporadic, in some cases there has been an autosomal dominant genetic component (associated with chromosome 14),16)(17) an autosomal recessive component,18) and chromosomal alterations, mainly in chromosomes 5 (5p deletion),18) (trisomy) and 22 (22q11.2 deletion), among others.19)(20
Classifications
The classifications used in HFM patients have evolved over time, covering more pathological aspects each time.
1. Classification system of Pruzansky (1969)
The first classification used in HFM patients was done by Samuel Pruzansky in 196921) using x-rays of the jaws of patients with this condition. In its classification, Pruzansky observed three types of mandibular hypoplasias, from a relatively full mandible (Grade I) to one very small and whose deformity worsened over time (Grade III) (Table 1-A). This classification includes only the description of the mandible, therefore, when used in HFM patients, it leaves out many aspects of the pathology.
3. Pruzansky′s classification modified by Kaban et al (1988)
In 1988, Kaban et al 22) changed Pruzansky′s classification by adding the description of deformations seen in temporomandibular joint teleradiography. The greatest difference appears in Grade II, which presents two sub-classifications depending on position of the glenoid cavity, which may be normal (IIA) or altered (IIB) (Table 1-B).
3. OMENS (1991)
Given the abundant phenotypic variability of this malformation, in 1991 the OMENS classification was proposed 23) based on three criteria, considering that an HFM classification should be inclusive and versatile, that the anatomic components should be separately analyzed, and that data should be expressed in a numerical scale to make them clinically useful. The OMENS abbreviation corresponds to its acronym for the five main manifestations of HFM: O: asymmetry of the orbit (Orbit); M: mandibular hypoplasia (Mandible); E: Deformity in the outer ear (Ear); N: Nerve involvement (Nerve); S: Deficiency in soft tissue (Soft Tissue) (Table 1-C).
3.1 Amendments to the OMENS
OMENS+ (1995): It was postulated in order to expand the phenotypic expression of HFM patients to extracranial alterations.24
Graphic representation of the OMENS+: a graphic representation of the OMENS+ was introduced in 2007 25) and later modified in 2011,26) in order to facilitate its understanding in clinical practice, teaching and the standardization of classification of HFM patients
4. CFDS (Craniofacial Deformity Scoring)27 ) (2001)
This corresponds to the sum of MDS (Mandibular Deformity Scoring) + CDS (Cranial Deformity Scoring). Through the use of computed tomography, different bone structures are analyzed and assigned values according to the extent to which they are involved; the maximum values are 16 points for MDS and 19 for CDS.
Clinical features
As this is an anomaly of the first and second branchial arches, most alterations are associated with structures developed from these arches,28) resenting wide phenotypic variation. The following structures are involved:
Jaw and TMJ: asymmetrical mandibular development for hypoplasia, absence of mandibular structures (condyle and ramus), absence or ankylosis of temporomandibular joint (TMJ). Recently published studies showed that HFM patients presented both mandibular and maxillary retrusion in comparison with the control group, along with an increase in the vertical component; these patterns were more marked in the affected side and increased according to severity.29) Mandibular height was always lower along growth, but the growth pattern was similar in both groups.30) In terms of TMJ, it has been observed that the degree of mandible dysplasia does not correspond to the degree of disk dysplasia, which vary among individuals; while the unaffected side does not present major alterations.31
Orbit: orbit dystopia (bad position), epibulbar dermoid, anophthalmia/microphthalmia, blepharoptosis, retinal or choroidal coloboma, among other less frequent anomalies.32
Ears: microtia, anotia, loss of hearing, disorders of the middle ear.
Cranial nerves: involvement of facial nerve and, in more severe cases, of the tgminal and hypoglossal nerves.
Dental: agenesis,7) dental hypoplasias,33) microdontia, and malocclusions. Delayed tooth development in HFM patients type IIB and III,34) with the most alterations in posterior teeth.35
Maxillofacial: labio-palatal fissure,36) macrostomia, hypoplasia of the facial thirds, occlusal plane inclination (highly variable in angle), hypoplasia of masticatory muscles,37) velopharyngeal insufficiency.38
Extracranial changes: primarily in kidney, lungs, heart, gastrointestinal, skeletal, and central nervous system (CNS).24
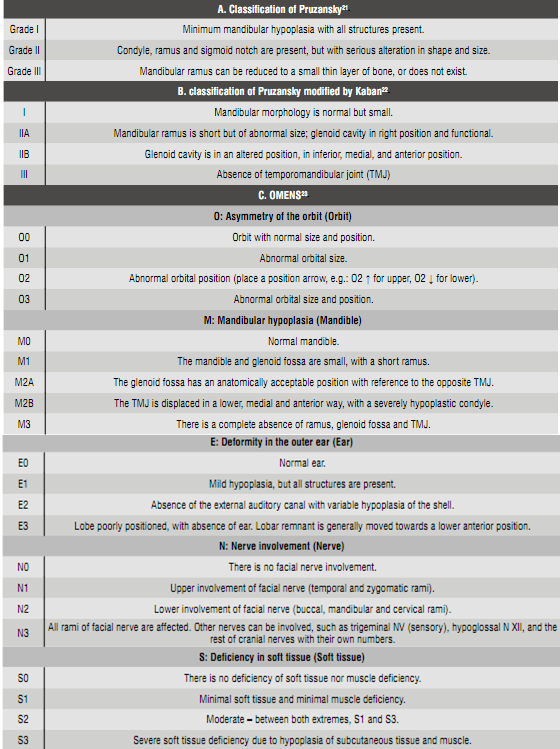
Because of this, it is necessary to establish the degree of involvement of the affected structures, both anatomically and functionally, in order to make timely referrals to the specialists in each of the involved areas. (Figure 1).
The photographs show patients with varying degrees of HFM. In image A, the patient presents a Grade I mandibular disorder, with slight deviation of the lower third. Image B corresponds to a Grade IIA alteration, presenting alteration of symmetry, both at the lower third and the orbits. Image C shows an alteration of the lower third, with full involvement of the right auricle, corresponding to a patient with a Grade IIB anomaly. Image D corresponds to a patient with a Grade III alteration of the left side.
Other aspects studied in HFM patients refer to an increased frequency in internalizing problems, poor social skills, and less acceptance by pairs - most commonly observed in women with young mothers at birth and additional alterations besides the mandibular-.39) An increased frequency of snoring and other sleep disorder symptoms has also been observed.40
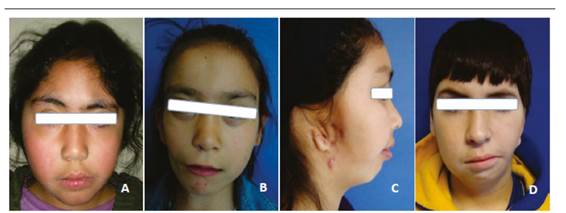
Although diagnosis is mainly clinical, various complementary tests allow a better analysis of this pathology. Panoramic radiography (Figure 2) allows an initial analysis of the structures of mandibular and maxillofacial structures, evaluating both sides in the same image. A profile teleradiography allows evaluating, through cephalometric analysis, the relations between maxilla and mandible, while front teleradiography allows observing the degree of asymmetry and mandibular deviation. An occlusal x-ray offers a clear view of the palatal vault in case of labio-palatal fissure.
These images show radiographs of HFM patients, all on the right side but with varying degrees of severity (Image A: Grade I; image B: Grade II, image C: Grade III). It can be observed that as severity increases, the symmetry between the rami and other jaw structures reduces, with total condyle loss in picture C.
An alternative way of evaluating HFM is through TC3D. While it allows a more realistic and detailed view, this technique requires applying a large amount of radiation on patients. A study by Takahashi et al in 2013 suggests careful use of both methods (panoramic radiography and TC3D), since the first had good reliability in cases of HFM patients in Grade I of Pruzansky, while reliability was low in Grade II patients, so the use of TC3D was preferable.41) Another tool used in HFM patients is photogrammetry, enabling a noninvasive approach in surgical planning.42
Photographic protocols are also useful in diagnosis and in evaluation of post-treatment advances. A study published in 2013 by Birgfeld et al 43) showed that it was feasible to establish the phenotype of HFM patients with both 2D and 3D imaging, which had several advantages and disadvantages. The strength of the 2D techniques was that clinicians find them easier to handle due to familiarity with them, while their weakness was that they were highly dependable on patient cooperation and photographer skills; in addition, they could not be rotated and needed proper photograph standardization, since slight inclinations made it difficult to correctly determine the degree of asymmetry. The advantage of 3D images was that they allowed rotating images and seeing the facial appearance from different angles; it was also faster to follow than a photographic protocol. Their main disadvantage was the lack of definition at the level of ear and eyelids, and hair interference, which resulted in loss of information.
Differential diagnosis
Hemimandibular hypoplasia with condylarcoronoid collapse: a condition not usually diagnosed at birth, with no soft tissue alterations and mainly characterized by chin deviation for hypoplasia of condyle, coronoid process, and mandibular ramus, always in the presence of temporal fossa (glenoid cavity).44)(45
Syndromes: being a clinically variable condition, HFM must be discarded by a geneticist of syndromes involving the maxillofacial complex. Syndromes such as Treacher-Collins, Miller-Dieker, Townes-Brocks, CHARGE, branquio-oto-renal, Parry-Romberg, among others, have similar characteristics to HFM, such as mandibular disorders, colobomas or eyelid malformations and abnormalities of the outer ear.
Treatment
In general, it is accepted that the best way to treat HFM patients is through interdisciplinary teams 46) whose diversity of specialties allows, through a joint effort, the appropriate treatment for each patient according to individual needs.
Treatment planning depends on type of malformation and severity in its expression, always taking into account patient and family expectations in terms of the results.
Mainly through plastic/orthognathic surgery and orthodontics, the treatment seeks to improve functionality, along with optimum facial symmetry, in order to:47)(48
1. Increase the size of the affected mandibular side and its associated soft tissue.
2. Create a joint simulating the TMJ in cases where it is absent.
3. Correct secondary deformities in maxilla.
4. Achieve functional occlusion, as well as aesthetic facial and dental appearance.
5. Improve and horizontalize the occlusal plane.
6. Achieve mouth opening if it is limited.
Conventional orthodontic treatment may initially include functional appliances with the use of rigid acrylic activators, which are individualized according to each case. These devices allow for expansion of affected tissue, taking advantage of patient′s physiological growth. Sometimes they can have height planes on the healthy side, allowing for vertical compensation of the affected area, always bearing in mind that facial midline should be centered with tooth midline. This can later be complemented with conventional fixed orthodontics.
Mandibular surgery
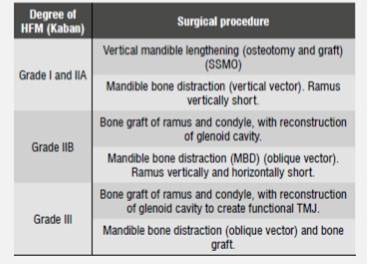
While the literature is broad in terms of techniques and surgical times, the treatment of HFM patients can be divided into two groups, according to the classification by Kaban-Prusansky: Grade I and Grade IIA patients are treated in the same way, while Grade IIB and Grade III patients are treated similarly among them but different to the first group.49) (Table 2).
Table 2 Surgical procedure according to hemifacial microsomia type following the classification by Kaban, according to Liu et al, 201249
Surgical management of the mandible is essential, and mandible bone distraction has several advantages over costochondral graft. These include: increase mandible vertical length, improve asymmetry of soft tissue, produce less blood loss, have better control of progress vector, and obtain a substantial improvement in the biomechanics of the lower mandible.50
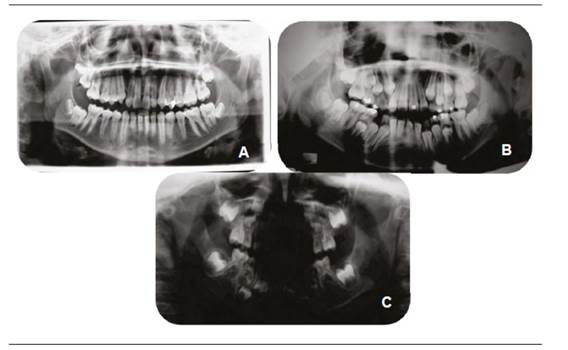
Bone distraction is based on the principle of tension-stress to allow elongation of bone and soft tissue from the controlled separation of bone segments (Figure 3). There are different types of distraction, which can be classified into two areas. According to location, distractors can be extraoral and intraoral; while, according to the amount of vectors with which they work, they can be univectorial or unidirectional or bi-directional (horizontal and vertical plane) or multidirectional (horizontal, vertical and transversal plane).51
Or thopantomographs of a 3-year-old patient with Grade II HFM during bone distraction with headgear. Image A corresponds to the initial x-ray. Image B corresponds to control x-ray after the distractor had been installed. Image C was taken one month after distraction. Note the change in distractor inclination, the increase in mandibular symmetry and the improved teeth relations.
The choice of distraction appliance depends on various factors, including patient′s age, the degree of malformation severity and the need for mobility of segments. In general, extraoral appliances require a less complex surgery, can achieve longer distraction distances, and allow handling the distraction in the three directions of space, but they leave scars on the skin, are more visible, and may be heavier, presenting patient discomfort. Intraoral devices are less visible and don′t leave facial scars, but have a limited range of motion in space, and their surgery is more complex for both insertion and removal. Re-absorbable devices have recently been developed, allowing for mandibular distraction in a single stage, and achieving progress of 15 to 30 mm.52) In anyways, the surgical simulation in a solid model can help achieve better mandible symmetry, especially in bone distraction with multivector.53
It is important to stress the need to take into account, besides patient′s growth state, his or her treatment needs, since they vary among patients, and therefore each must be analyzed as a unique case.
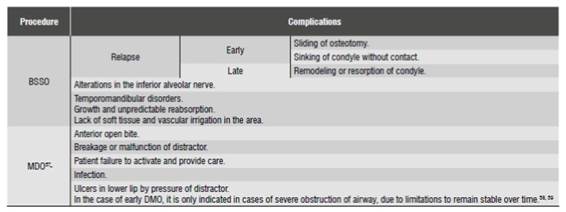
Complications
A 2009 literature review 54) compared the stability and complications of bone distraction and the costochondral graft. It found out that both techniques have a similar relapse rate when performing mandibular advancement of 6 to 10 mm. The main complications are shown in (Table 3). While this review suggests that mandibular bone distraction is less susceptible to major complications, it also points out the it may produce minor complications that also cause patient morbidity, in addition to being affected by other factors such as a long phase of consolidation, distractor cost, patient compliance in activating the distractor and the need for a second surgery to remove the distractor.
Other surgeries
Being a malformation with abundant phenotypic variation, HFM patients may need other surgeries, depending on the involved structures. A review of craniofacial microsomia performed by Birgfeld and Heike in 2012 55) shows a timeline as a guide in the medical and surgical management of HFM patients, proposed by members of the Seattle Children′s Craniofacial Center, monitoring its progress from birth to adulthood. Depending on patient, it may be necessary:
Orbit:It may involve surgeries of epibulbar dermoid and eyelid coloboma. In cases of changes in size or position of the orbit, the replacement is done around the age of 3 to 4 years.
Ears:Tympanic ventilation tubes (clamps). In case of alteration of the outer ear, surgery varies according to the involvement of pinna, from remodeling cartilage in the event of slight hypoplasias to the complete reconstruction through autologous or alloplastic grafts, such as porous high-density polyethylene. In patients with hearing loss, the possibility of repairing atresia of the auditory canal should be evaluated.
Facial nerve:in cases of nerve palsy, the area and degree of involvement should be evaluated; this involvement may be upper (affects the temporal and zygomatic area), lower (buccal, mandibular and cervical area), or total. In the case of oral involvement, the need for facial reanimation should be evaluated. In case of alteration of eyelid movement, some type of treatment should be considered in order to prevent corneal keratitis by exposure.
Soft tissue:Closure of orofacial fissures, correction of airway, surgery of soft tissue augmentation, and fat grafts.
In Chile there are no published treatment protocols for hemifacial microsomia, a pathology that is not covered by the public health system, so most patients have their treatments in privet offices and therefore are conditioned by various factors such as access to specialists with knowledge in HFM, cost of procedures, or health prevision, among others.
In Latin America there is only one follow-up protocol 56) published in Argentina, but there is no treatment protocol.
CONCLUSIONS
Hemifacial microsomia is a heterogeneous, variable disease of unique expression in each subject, both in its etiology and severity and therefore in its treatment. Being an alteration of wide spectrum, it affects various structures of the individual according to its severity. This is why a very wellcoordinated interdisciplinary work is vital in these patients, since they may even have psychosocial and extracranial alterations which should be timely explored and treated.











 text in
text in