INTRODUCTION
Calcium silicate cements are gradually making their way through the various materials used in restorative dentistry. While it is true that they have long been used in endodontics, their introduction in restorative dentistry is more recent. Mineral Trioxide Aggregate (MTA) was the first of this type of materials to be developed (patented in 1995). As a result of the favorable properties of biocompatibility and bioactivity of this first material, many manufacturers developed other MTA-like products, such as MTA Angelus (Angelus Soluções Odontológicas, Brazil) and Endo CPM Sealer (Egeo, Argentina).1) These materials are largely used in endodontic treatments; however, they can also be used in restorative dentistry, including direct pulp capping.1)(2
Later, in 2011, a new material appeared in the market: BiodentineTM(Septodont, Saint Maur des Fossés, France), which is indicated as a replacement for both coronal and root dentin.3) The quick hardening of this cement, in comparison with previous calcium silicates, and its improved mechanical properties made it suitable for definitive restorations in replacing dentin and as a temporary cement to restore enamel.3) Other materials, such as TheraCal LC (Bisco Inc., Schamburg, IL, USA), have been developed more recently suggesting the use of calcium silicates mixed with composite resins, which can control hardening times since they are light-curing materials.
One of the greatest advantages of calcium silicates is their so-called bioactivity property. Bioactive materials are defined as those that "trigger a biological response in the tissue-material interface, resulting in the formation of bonding between material and tissue".4)(5) This is evident in the favorable responses observed when the material is in contact with soft tissues such as pulp and periodontal tissues, or with hard tissues such as dentin.6)(7)(8
Research shows that these cements can produce strong bonding with dentin through an area of mineral infiltration, with formation of mineral tags and diffusion of calcium and silicon to dentin.9)(10) In addition, in contact with pulp tissue, the material can stimulate dentin bridge formation.11) This is why the study of these materials is of particular interest to restorative dentistry, due to their potential use as restorative materials in case of deep dentin cavities, as well as in direct and indirect pulp capping therapies.
Since calcium silicate cements have expanded their range of indications, including some for restorative dentistry, and due to the emergence of new silicate calcium-based materials with important variations in their compositions, it is necessary to review the available scientific literature that assess their use in these applications, due to the lack of reviews focusing on this particular topic. Therefore, this review article aims to evaluate the available information on calcium silicate cements, focusing on their possible applications in restorative dentistry. Thus, it seeks to update the clinicians′ knowledge about calcium silicate cements, helping them make more informed clinical decisions.
We conducted a topic review by searching on the Pubmed/Medline and Scielo databases using the following key words: calcium silicate cement, tricalcium silicate cement, Mineral Trioxide Aggregate, BiodentineTM, TheraCal LC, andbioactive cements. In addition, technical information provided by manufacturers of these cements was collected. Data analysis involved reviewing the abstracts of available articles, and only those that were considered relevant to the subject matter were included.
MINERAL TRIOXIDE AGGREGATE
Mineral Trioxide Aggregate (MTA) was the first calcium silicate developed for dental use; it was developed and patented by Torabinejad and White in 1995.12) Its main component is Portland cement type I (calcium silicate), known as regular Portland cement used in construction, which is added bismuth oxide (Bi2O3) to provide it with radiopacity.12
Composition and instructions for use
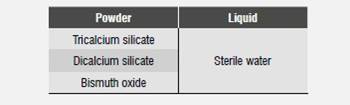
The original MTA formula was developed at the University of Loma Linda, United States, and was manufactured by Dentsply International (ProRoot MTA and Tooth-Colored MTA; Dentsply-Tulsa Dental, Tulsa-USA; Dentsply-Johnson City- USA). However, various similar products have been manufactured by other companies.1) Several studies have provided detailed information on the components of the main types of MTA, ProRoot MTA (Grey MTA or GMTA) and Tooth-Colored MTA (White MTA or WMTA).13) The main components of GMTA are described in Table 1, while the components of the white version, WMTA, are tricalcium silicate and oxide bismuth.13) Studies comparing their composition have concluded that the difference in color between these materials is due to the lack of iron compounds in the WMTA formula. The observations have also found smaller particles in WMTA compared with GMTA, suggesting that this may be connected to the easier handling of WMTA.13)(14)(15
These cements are prepared by mixing MTA powder with sterile water in a 3:1 ratio.16) A plastic or metal spatula is used to mix the cement in a glass lab, and the mix can be applied with an instrument such as a plastic or metal amalgam carrier to bring the material to the application site.16
Curing reaction
Mixing the powder with sterile water produces a colloidal gel which soon solidifies.1 During this mixing, a hydration reaction occurs with the components, leading to the formation of calcium silicate hydrate (C-S-H) and calcium hydroxide as by-products.17) Once the mixture starts, its pH value increases sharply, reaching to pH 12 after 20 m, which remains for three hours.18)(19) Camilleri has studied the chemical changes that occur when the cement hydrates. It has been observed that a high proportion of calcium ions is released quickly, due to the dissolution of calcium hydroxide to a progressive decalcification of C-S-H. This occurs more rapidly than the release of silica and bismuth. It is thought that high levels of calcium released are connected to the biocompatibility of the material, since the elution of calcium hydroxide induces cell proliferationin vitro.17
The curing time of the original version of MTA, GMTA, is 165 (+/− 5) m;18) while WMTA takes 70 (+/− 8.5) m, with a working time of 5 (+/− 0.79) m.20) This extended curing time is one of the biggest disadvantages of this type of material, and is one of the reasons why it cannot be used in single-session procedures.2) Generally, clinicians should confirm the material′s curing time in a second session before moving to the next step.
Applications in restorative dentistry
Among the numerous applications in endodontic treatments, MTA has also been proposed in direct pulp capping.2) The technique for performing this therapy in vital teeth has been fully described by Whiterspoon.21
Direct coating
Several review articles on the clinical applications of MTA have been published.2)(21)(22)(23)(24) In 2010, Parirokh and Torabinejad conducted a review suggesting that MTA is a promising material to preserve pulp vitality when used in direct pulp capping.2) The authors state that this seems to be the material of choice for direct capping therapies, compared with other available materials for the treatment of permanent teeth.2) In 2011, Aguilar and Linsuwanont published a systematic review on pulp therapy in permanent teeth with pulp exposure due to caries and treated with MTA and calcium hydroxide.22) They found out that both materials can provide satisfactory results in pulp therapies, such as direct pulp capping and total or partial pulpotomy. Success rates after 3 year were high: 72.9% for direct pulp capping (in patients aged 6 to 10 years), 99.4% for partial pulpotomy (in patients aged 6 to 27 years), and 99.3% for total pulpotomy (in patients aged 6 to 70 years).22) However, the authors also stated that the evidence available at the time provided inconclusive information and they highlighted the need for more high-quality studies.22
These revisions were followed by four publications of trials comparing MTA and calcium hydroxide (a material generally used in vital pulp therapies in permanent teeth), most of which found better results for MTA.25)(26)(27)(28) Mente et al assessed 149 patients (with an average of 27 months follow-up) who were treated with direct capping following pulp exposure, using calcium hydroxide and MTA.27) They observed a higher rate of success with MTA (78%) compared with calcium hydroxide (60%), concluding that MTA seems to be more effective in maintaining pulp vitality after direct capping.27 Similar results were obtained by Hilton et al in their randomized clinical study, finding out a lower probability of failure in teeth treated with MTA (19.7%) compared with calcium hydroxide (31.5%).25) Their study included a large sample of 376 patients who were monitored for up to 2 years.25) On the other hand, Chailertvanitkul et al found no difference in terms of success rate when performing direct capping following pulp exposure with MTA and calcium hydroxide, but they did find a tendency to a higher probability of failure in pulp exposure greater than 5 mm2, with a 2-year follow-up.26) Leye et al found no significant differences in survival rates with MTA and calcium hydroxide at 6 months, but they did find differences at 3 months, with more favorable results for MTA.28) A clinical study has also been published evaluating the preservation of the vitality of teeth treated with MTA in direct capping.29) The success rate (conservation of vitality) after 3.6 (+/−1.1) years was 91.3%.29
The scientific evidence of the use of MTA in direct pulp capping therapies has been growing slowly. However, despite the favorable results for MTA, the amount of high-quality clinical studies is still low in this area, with follow-ups in the short and medium term.
BIODENTINE TM
BiodentineTM is a cement-based calcium silicate that has been advertised as "the first all-in-one material" to be used whenever dentin has been damaged.30) This material has been developed in an effort to produce a calcium silicate with better mechanical properties31) and hardening times.32
Composition and instructions for use
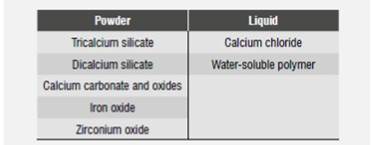
BiodentineTM comes as a capsule containing powder and a liquid contained in a vial. According to the mixing instructions, the contents of the vial should be squeezed into the capsule and then mixed in an amalgamator for 30 s. Depending on preference, the contents of the capsule is applied with a porta amalgam, a spatula, or a device such as the Root Canal Messing Gun.33 Table 2 shows the components as stated by the manufacturer.32
According to the manufacturer, the Active Biosilicate TechnologyTMused to produce BiodentineTMensures the purity of calcium silicate, as opposed to other calcium silicate cements based on Portland cement which contain non-purified mixtures with low concentrations of metal impurities.32) However, recent studies have found remains of arsenic, lead, and chromium in BiodentineTM.34) Moreover, the found levels of arsenic are higher than those allowed by ISO 9917. Nevertheless, the same components have been reported for MTA, but since the release in the physiological solution is minimal, they have been considered safe.34
The manufacturer has suggested that this material′s reduced curing time (12 m) compared to traditional calcium silicates such as MTA (70 ±8,5 m)20) is due to the smaller size of the powder particles, thus allowing a greater reaction area. In addition, the calcium chloride added to the liquid has proven to be a powerful accelerator of reaction in these materials.35)(36) The manufacturer also states that the material′s best mechanical properties are due to the lack of impurities, along with the addition of calcium carbonate powder and the optimal density of the powder obtained in the mix.32) The watersoluble polymer probably plays an important role in achieving better powder density, since an easyto- handle mix is obtained with a smaller amount of water.32) Finally, it has been supposed that zirconium oxide is added in order to provide it with radiopacity, since it is has been used in other materials for the same purpose.37) This is another important difference with MTA, where radiopacity is provided by means of oxide bismuth-a compound that according to some authors has an unwanted effect on the material-.38
Curing reaction
The curing reaction of BiodentineTMis similar to that of MTA, with production of hydrated calcium silicates and calcium hydroxide as by-products,39but the speed of reaction is greater in BiodentineTM.40)(41
The initial curing reaction takes about 12 m.41) However, impedance spectroscopy has shown that the reaction continues for up to 14 days.42) The study of Villat et al suggests that the complete hydration reaction of this silicate is much slower than that observed in the acid-base reaction of glass ionomer cements, concluding that this reaction could continue for months, extending ion exchange, decreasing porosity, and increasing the material′s mechanical properties.42
Treatment
BiodentineTMis indicated as a substitute for dentin in both the coronal portion and the root.32) Indications for restorative dentistry include:
Temporary restoration of enamel
Final restoration of dentin
Restoration of lesions of large and/or deep cavities (sandwich technique)
Restoration of deep cervical or root lesions
Direct and indirect pulp capping
The manufacturer indicates that applying the product does not require any prior treatment and that, once hardened, the cement should be treated as if it were healthy dentin. In the case of a sandwich technique using this material, it has been recommended to fully restore the cavity in the first session, remove the outer part after one week to six months and cover it with composite resin.33
Direct pulp capping
Only one clinical study assessing BiodentineTMas a restorative material in direct pulp capping has been published to date. The study by Nowicka et al involved drilling pulp premolars extracted for orthodontic purposes capping with BiodentineTM(n = 11) and MTA (n = 11). After 6 weeks, most premolars showed formation of full dentin bridge, with absence of pulp inflammatory response; no significant differences were found between BiodentineTMand MTA during the observation period.11
Other articles have evaluated this material in animal models and in extracted molars. Tran et al conducted a study in rats also showing the consistent formation of dentin bridge in pulp cappings made with BiodentineTMand MTA.43
In these cases, the formed bridge is located in the affected area, with an ortodentine type of organization, in contrast to what was observed in treatments performed with calcium hydroxide, which showed cell inclusions similar to osteodentine.43
In their study, Laurent et al used healthy premolars recently extracted, which were kept in a culture and subjected to direct capping procedures with BiodentineTM.8) In all the evaluated premolars (n = 15), they noted the formation of mineralization foci, which increased in size until day 28-date of the last observation-. They also noticed the expression of markers of mineralization, suggesting that the material is capable of inducing the differentiation of odontoblast cells, involved in the formation of dentin tissue.8
However, the level of evidence in studies in animals or in exvivo models is smaller than that achieved in clinical trials. Therefore, it is necessary to conduct additional clinical trials to provide more evidence on the use of this material in direct pulp capping.
Indirect pulp capping
A randomized clinical study recently evaluated the use of BiodentineTM in indirect pulp capping. The study analyzed 72 restorations (36 made with BiodentineTM and 36 with glass ionomer), with a follow-up of up to one year, finding out no differences between the materials when measuring the clinical efficacy of pulp vitality conservation.44) However, the authors noted that most teeth with apical radiotransparency (which was not detected at baseline with periapical x-rays but later with computed tomography) that decreased in size or were eliminated were treated with BiodentineTM,44) while most recent lesions or their progression were found in teeth treated with glass ionomer.44) These results were attributed to the bioactive characteristics of BiodentineTM, which have been reported from in vitro studies.6)(7)(8)(45
Permanent restoration of dentin and temporary restoration of enamel
Only one clinical study using BiodentineTMas a restorative material (of enamel and dentine) has been published to date.46) This clinical, multicentered, randomized study with a three-year follow-up has only published the results obtained during the first year.46) Class I and Class II restorations (n = 397) were performed with BiodentineTMand composite resin.46) The initial assessment of the product shows very satisfactory results in terms of anatomical shape, marginal adaptation, and proximal contacts; however, the composite resin restorations showed better clinical behavior in these parameters after six months. This is why this study recommends that after 6 months it is necessary to remove the outermost layer of BiodentineTMand to restore with composite resin, leaving it only as permanent replacement of dentin and temporary replacement of enamel.46
THERACAL LC
TheraCal LC is a resin-modified calcium silicate cement developed by Bisco Inc. to be used as a barrier and protection of the pulp-dentin complex.47It comes in a syringe containing a photo-curable paste composed of calcium oxide, particles of calcium silicate, glass of strontium, barium sulfate, silica, barium zirconate, and resin (BisGMA and PEGDMA). According to the manufacturer, it is indicated for direct and indirect pulp capping applied as a cavity liner.47
In vitro studies have examined its physical and chemical properties.48)(49)(50) Camilleri noted that, just as BiodentineTM, TheraCal LC allows calcium phosphates to deposit on its surface when in contact with a saline solution;50) however, the release of calcium ions is significantly lower that than of BiodentineTM.49)(50) Gandolfi has demonstrated that TheraCal LC solubility is less than that of MTA and calcium hydroxide; in addition, it has a weak radiopacity (less than required by standard ISO 6976) and can be light-cured in thickness of 1.7 mm.48
Since this material has been recently released, there are no clinical studies evaluating its behavior, and so far there is only one published study in animals. Cannon et al conducted a study in primates performing direct pulp capping with TheraCal LC. The authors noted that teeth treated with this material had way more frequent dentin bridge formation, compared with calcium hydroxide and glass ionomer.51
DISCUSSION
Calcium silicates have long been part of the variety of dental materials available in the market; however, their use in restorative dentistry used to be limited to a few applications. Mineral Trioxide Aggregate (MTA), due to its excellent biocompatibility and bioactivity properties and low mechanical properties, is indicated for direct capping.1)(2) In comparing it with alternative materials for these therapies, the scientific evidence shows favorable results when using it for these indications. Both systematic reviews and randomized clinical trials agree that this material is effective in maintaining vital teeth, with consistent formation of dentin bridge.2)(22)(26)(27) The success rates of therapies using this material are comparable (and in some studies even higher) to conventional materials, such as calcium hydroxide (tempered in the studies by Hilton et al, Chailertvanitkul et al, and Leye Benoist et al, and non-tempered in the study by Mente et al).25)(26)(27)(28) However, it is necessary to conduct more long-term clinical studies in order to provide further evidence.
The development of BiodentineTMexpanded the indications of calcium silicates in restorative dentistry. The composition of this material is similar to that of MTA but with significant variations that imply changes in its physical properties.32) The alleged best mechanical properties of BiodentineTM, as well as its reduced curing time, allows it to be used in a wide range of indications. It has been suggested as a material for dentin replacement in Class I, II and V cavities, and as replacement of enamel on a temporary basis (up to 6 months). These applications of BiodentineTMare brand new within calcium silicates, so further assessment is needed.
The results of clinical studies are promising. BiodentineTM, in addition to having faster curing times compared to other calcium silicates, is easy to handling as it comes in capsules, allowing its clean and accurate application on teeth. This material makes a very good alternative for the treatment of deep dental caries, including cases with reversible pulpal inflammation already occurring. Due to its bioactive properties, BiodentineTMmay provide appropriate pulp-dentin sealing, favoring pulp response and changing the conditions of tissues affected by tooth decay.
TheraCal LC is a cement of recent availability in the market; as an advantage, it can be photocurable.47) The effects of this incorporation of resin to a calcium silicate cement have been explored in some in vitro studies;48)(49)(50) however, no clinical studies have been reported to date.
The scientific evidence on calcium silicate cements is in general focused on materials that have been available for a longer time, such as MTA.11)(44)( 46) There are no many clinical studies on newer calcium silicate cements.11)(44) This prevents from having more consistent information to determine their clinical efficacy. This level of evidence is certainly needed in order to make conclusions on these materials; it is therefore necessary to conduct evaluations through randomized clinical trials, in order to provide clinicians with accurate information for decision making.
CONCLUSIONS
Calcium silicates are alternative dental materials that can be used in direct and indirect capping, cavitary liner, dentin replacement in class I, II and V cavities, and as semi-permanent restorations of enamel.
Indications for direct and indirect capping are supported by clinical studies, especially in the case of MTA for direct capping. New applications proposed for these materials, such as replacement of dentin in class I, II and V cavities have still insufficient clinical evidence; However, in vitro studies show promising results.
The biocompatibility and bioactivity properties make of calcium silicates one of the restorative materials that offer a more favorable response by pulp tissue.











 text in
text in