INTRODUCTION
It has been 36 years since the World Health Organization (WHO) first set forth global goals for oral health and there is still much work to be done. In 1989, the same organization recommended promoting oral health as a public health issue.1) Consequently, many countries set up strategies to reduce oral diseases. Although according to global reports2) public health efforts have been effective in controlling oral diseases, dental caries remains one of the most prevalent chronic diseases.3) Dental caries may affect basic functions like food intake and nutrition, and can influence the psycho-social life of patients.4) It can also produce tooth loss and serious diseases such as bacterial endocarditis.5
In Colombia, the most recent Estudio Nacional de Salud Bucal ENSAB IV (2014) showed a decrease in caries prevalence compared to the previous report, especially in the population aged 12 years (going from 57% to 37%) and in the population aged 20 to 34 years (from 75% to 53%). Despite these results, caries remains the most frequent disease in the earlier years, since 33.3% of children aged 1, 3 and 5 years already have experience of this disease, with a prevalence of 5.9%, 43.7% and 52.2% respectively. This shows the need for strategies to complement the existing ones, to achieve the eradication or a greater control of this disease.6
Dental caries is a disease caused by a shift in pH balance, resulting in the formation of a biofilm composed mainly of cariogenic microflora. The interaction between this microflora and host factors conditions the deceleration or acceleration of the disease.7)(8)(9
Imbalance or dysbiosis of the oral microflora make this disease an endogenous infection, which is not transmissible, as previously believed.10)(11) In recent years, several research groups have focused their efforts on the study of oral microbiota,12)(13)(14) identifying microorganisms involved in cariogenic processes, microorganisms of the healthy oral cavity, their metabolic processes, and the interactions between microorganisms and host. Some biotechnology tools have been tried based on all this research, including probiotics,15)(16) intended for a selective control of the etiological agents of caries and the maintenance of oral homeostasis. The WHO/FAO have accepted the definition of probiotics as “live microorganisms, mainly bacteria, that are safe for human consumption, and when administered in adequate amounts confer a health benefit on the host”.17
The objective of this topic review is to present probiotics as a strategy for caries control within the context of microbiology and the maintenance of oral homeostasis, as supported by studies conducted on different bacterial strains that comply with this characteristic.
MICROBIOLOGY OF THE ORAL CAVITY
It has been estimated that there are over a thousand different species of bacteria in the oral cavity as potential colonizers.18)(19)(20)(21)(22) Only 280 species have been isolated and identified, but using molecular techniques based on the cloning of gene 16S RNA, about 600 species or phylotypes have been identified.19) Most of these bacteria are commensals, maintaining the homeostasis of the ecosystem, while others are involved in the development of pathologies.23)(24)(25
The types and proportions of bacteria vary according to the conditions of the oral cavity.12)(13)(14)(26) These differences are evident between a healthy oral cavity and one with some kind of pathology, such as caries,27)(28)(29, periodontitis, (30) or cancer.31)(32
The healthy oral cavity is characterized by a microflora dominated by phylum Firmicutes (genus Streptococcus, family Veillonellaceae, genus Granulicatella), Proteobacteria (genus Neisseria, Haemophilus), Actinobacteria (genus Corynebacterium, Rothia, Actinomyces), Bacteroidetes (genus Prevotella, Capnocytophaga, Porphyromona) and Fusobacteria (genus Fusobacterium).33) In a study based on dental plaque from caries-free children, the authors identified an overabundance of Streptococcus parasanguinis, Abiotrophia defectiva, Streptococcus mitis, Streptococcus oralis, and Streptococcus sanguinis.( 34) Other bacterial communities associated with caries-free conditions are Cordiobacterium, Rothia, Kingella Aggregatibacter or Mannheimia, some of which, despite being abundant, are not fully known as they belong to groups that cannot be grown in laboratory.35) The findings of a microbial community related with non-cariogenic status support the idea of using bacteria associated to the healthy status as probiotics to prevent oral diseases.28
Regarding microorganisms in oral cavity with caries, some bacteria found in high proportions in carious lesions were identified during the 1960’s and 1970’s.36) During this period, the importance of Streptococcus mutans and related organisms (S. sobrinus, S. cricetus, S. rattus, S. downii, and S. macacae) was established in the etiology of dental caries.27) Another group of bacteria found in high proportions in carious lesions are the acidogenic and acid tolerant lactobacilli which, like S. mutans, transform fermentable carbohydrates into lactic acid, producing tooth demineralization.37
Today, with the advances in molecular techniques, such as PCR, pyrosequencing and the sequencing of 16S rRNA, as well as with the establishment of genomic databases like the Human Oral Microbiome Database (HOMD)38) and OSU CORE Database,39) more microorganisms are known to be involved in the development of dental caries.10) The identified bacteria include Selenomonas, Neisseria, a variety of species of Streptococcus, Bifidobacterium, Propionibacterium, Scardoviawiggsiae, Veillonella parvula, Veillonella atypica, Megasphaera micronuciformis, Fusobacterium periodontium, Achromobacterxylosoxidans, and Actinomycesgerensceriae.( 27)(28)(29
The great progress achieved in characterizing microorganisms in the oral cavity has not been sufficient to develop strategies to control the main pathologies. This is due to the complexity of the ecosystem and because understanding the interactions between these microorganisms and the host is critical.33) The development of “omics” sciences (metatranscriptomics, proteomics, or metabolomics) has enabled a better understanding of these interactions. In fact, it has been concluded that the detection of metabolic profiles associated with the disease provides more information than the composition of microorganisms.10) As Simon-Soro et al put it, research must focus on intercepting the functions related with the onset and progression of the disease, such as the strategies to regulate pH, the disruption of molecules of adhesion among microorganisms or between microorganisms and the host, or the use of antimicrobial strategies like probiotics, which modify the microbial interaction and therefore the cariogenic effects.10
STUDIES WITH PROBIOTICS IN THE ORAL CAVITY
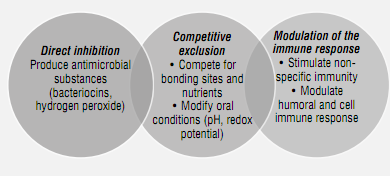
Probiotics are widely studied and used for gastrointestinal problems, such as diarrhea by consumption of antibiotics, infection by Helicobacter pylori, management of intolerance to lactose, irritable colon syndrome, and colitis, as well as for urogenital and allergic diseases, to name just a few.40)(41)(42) Their use is also common in daily products in order to improve the functioning of the digestive tract and to boost the immune system.43) Regarding probiotics for oral health, they are less frequently used despite the findings of several studies19)(44)(46)(47) and the development of commercial products with this component.48) Also, probiotics have several mechanisms of action to control oral pathogens (Figure 1)
Source: by the author based on the literature 49)(50))
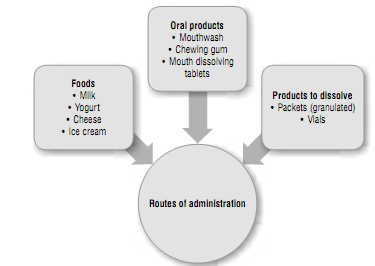
Several bacterial strains have been studied as probiotics, either isolated or in combination, as well as the doses used in studies, intervention periods, populations, experimental designs (Table 1), and routes of administration (Figure 2). The results often include a decrease in S. mutans count in saliva or plaque, but few studies conduct dental clinical examinations to assess treatment (Table 1). This is confirmed by a meta-analysis conducted by Laleman et al in 2013, claiming that the scientific evidence demonstrating caries control is insufficient.15) However, the results of studies describing the effect of probiotics in the index of carious lesions44)(47) or dental plaque51)(52) are promising (Table 1).
Table 1 Studies carried out with probiotic bacteria to reduce Streptoccus mutans and control caries
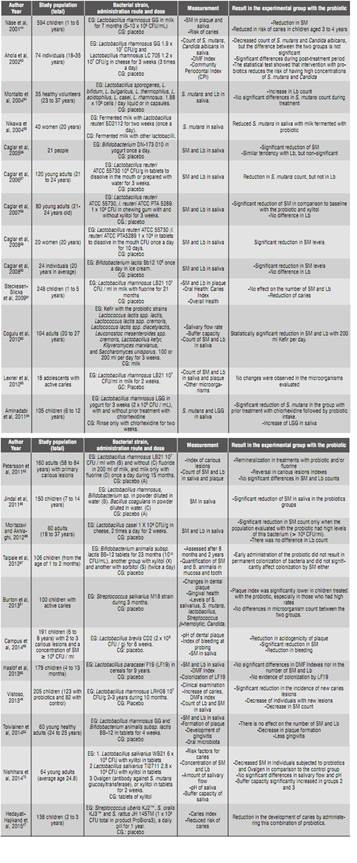
SM: streptococcus of the mutans group Lb: Lactobacillus CFU: Colony-Forming Units: EG: experimental group CG: control group.
Source: By the author based on the literature ( 15)(16)(64)(65)(66)(67)(68)(69)(70)(71
The probiotics most commonly evaluated so far in the oral cavity are isolated microbial strains, mainly of the digestive tract, like the ones belonging to the genus Lactobacillus (L. rhamnosus, L. reuteri, L. casei, L. brevis, L. paracasei, L. acidophilus, L. plantarum) and Bifidobacterium (B. bifidum, B. longum, B. lactis, B. animalis, B. infantis)37)(49 (Table 1). These bacteria have proven their safety for many years, allowing clinical studies and the development of commercial products, with the added value that some have been identified in healthy individuals (DMF = 0) (L. rhamnosus, L. plantarum, L. acidophilus, L. brevis, L. paracasei),72 suggesting their role in micro-ecologic balance. As a drawback to their use, some studies have shown their poor colonization capacity in the oral cavity,73)(74) especially in hard tissues,72) in addition to acidogenic properties of some strains and their presence in carious regions,75) suggesting the opposite effect to that expected with probiotics.
One of the most commonly studied probiotic bacteria in the oral cavity was isolated by Gorbach and Godin in 1983 from digestive tract of a healthy adult.76) It was named Lactobacillus rhamnosus GG (ATCC53103) and is characterized by inhibiting, by means of bacteriocins, a wide variety of human pathogenic bacteria,77) including S. mutans, S. sobrinus, Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia.( 78)(79) In addition, this bacterium colonizes the microcosm of saliva, and has no cariogenic effect.79) This bacterium was used to conduct one of the most complete clinical studies in children (aged 1 to 6 years), reducing the incidence of caries in the age of 3 to 4 years.44
Lactobacillus reuteri is another bacterium that has been commonly studied, and is usually found in the human gastrointestinal tract. It produces bacteriocins that inhibit Gram-positive bacteria as Bacillus cereus, Staphylococcus aureus and Listeria monocytogenes and Gram-negative bacteria such as E. coli, Yersinia enterocolitica and Pseudomonas fluorescens.55) In the oral cavity, it has been shown to inhibit S. mutans in saliva, and Tannerella forsythia and S. gordonii in vitro.80) The strains most frequently studied in the oral cavity are l. reuteri ATCC 55730, isolated in 1990 from a Peruvian mother’s breast milk, and l. reuteri ATCC 5289, isolated from the oral cavity of a Japanese woman.81) These bacteria showed suitable characteristics for colonization and maintenance of pH in the oral cavity. In 2012, Jalasvuori et al showed that in vitro the strains kept a pH over 7 during 4 hours of incubation in presence of glucose and sufficient amount of arginine, showing the arginolytic nature of these bacteria.81) Strain ATCC 5289 is the most efficient to keep an alkaline pH, and has a greater colonizing potential due to its capacity of adhesion and formation of biofilm.81)(82
Species of the genus Streptococcus, such as S. salivarius, S. sanguinis and S. oligofermentans, are specific to the oral cavity and have been studied as possible probiotics. S. salivarius is one of the earliest colonizers of the epithelium surface in the human mouth and nasopharynx, and its primary habitat is the dorsum of the healthy tongue; the K12 strain was isolated from a healthy child’s saliva, and is characterized by producing bacteriocins which inhibit the growth of Gram-negative bacteria associated with periodontitis and halitosis
83) and Gram-positive bacteria like S. mutans. In addition, it has the property of producing ammonium from arginine and urea, through the expression of the gene of urease in conditions of acidic pH and excess carbohydrates.84
The role of S. oligofermentans as a probiotic was discovered during a clinical trial noting an inverse relationship in the amount of this bacteria with respect to S. mutans in dental plaque.85) An in vitro study confirmed the inhibitory effect of this bacterium, due to the production of hydrogen peroxide from lactic acid, suggesting its inhibitory capacity in the presence of bacteria producers of lactic acid.86
S. sanguinis, just like S. mutans, is one of the primary colonizers of dental plaque, but with an inverse relationship in quantity, suggesting an antagonism between these two bacteria.87) A subsequent study showed that this antagonism was due to the production of hydrogen peroxide by S. sanguninis on the one hand, and the production of bacteriocins by S. mutans, on the other.88) The inhibitory capacity of a bacterium on the another is determined by the ecological factors of the host and by the survival mechanisms of the bacteria in the oral cavity, due to the regulation of inhibitory compounds by the environmental conditions and the juxtaposition of the two species.89
IN SEARCH OF THE IDEAL BACTERIA AS PROBIOTICS
Various research groups90)(91)(92)(93)(94 have conducted studies aiming to isolate, identify, and assess bacterial strains complying with the characteristics of probiotics in the oral cavity (Table 2). One of such studies is that by Strahinic et al, who searched for strains of Lactobacillus sp. with probiotic characteristics in human oral cavity, identifying l. salivarius BGH01 and l. gasseri BGH089.90) These strains showed antagonism against human pathogenic bacteria (Staphylococcus auereus, Enterococcus faecalis, Micrococcus flavus, Salmonella enteritidis), including S. mutans. A subsequent study showed that L. salivarius BGH01 produces more than one bacteriocin; of these, LS1 and LS2 have already been isolated and evaluated.95
Another study, focused on finding lactic acid bacteria in saliva from healthy children, identified 11 bacteria strains that met the criteria defined by the researchers, such as ability of adherence to oral tissues and aggregation to form biofilm, antagonism against pathogens, bacterial genetic identification, absence of acids and malodorous volatile compounds, and absence of resistance to antibiotics.91
Camelo-Castillo et al identified two strains of bacteria that they named Streptococcus dentisani in the dental plaque of individuals who have never had dental caries. This bacterium belongs to the mitis group, but was grouped with a new phylogenetic branch. Through the analysis of genomic and metabolic characteristics, they confirmed it belongs to a new species of the genus Streptococcus. As probiotic characteristics, S. dentisani has antimicrobial activity against S. mutans in in vitro conditions, and produces ammonium by arginine metabolism, controlling the pH of the oral cavity and thus preventing the colonization of strains related to the development of caries.92
To identify bacteria with probiotic characteristics, Wu et al analyzed bacterial strains of Lactobacillus salivarius with antagonistic activity against S. mutans. They studied 64 strains and found two, K35 and K43, which significantly inhibited the formation of biofilm of S. mutans. Subsequent in vitro studies confirmed that they had strong bactericidal activity against S. mutans.93
In 2015, Terai et al selected, from dental plaque and tongue of healthy individuals, bacteria of the genus Lactobacillus and Streptococcus which did not produce volatile sulfur compounds (VSCs) or water-insoluble glucans, with high antibacterial activity against caries and periodontitis-causing bacteria as well as high activity of adherence to oral epithelial cells and hydroxyapatite in vitro. The authors selected L. crispatus YIT 12319, L. fermentum YIT12320, L. gasseri YIT 12321 and S. mitis YIT 12322 for complying with the abovementioned characteristics, and for not having cariogenic risk or causing infective endocarditis.94
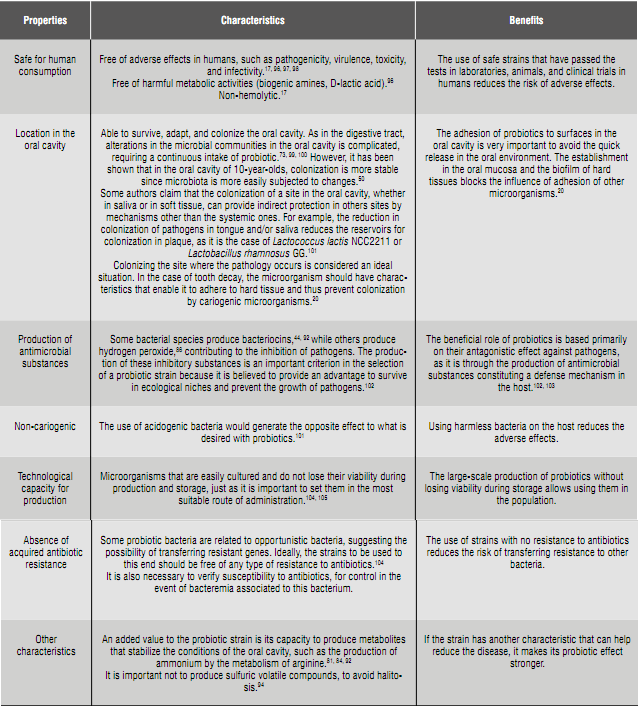
In addition to presenting findings related to the evaluation for probiotic characteristics, some of the studies 91)(94 show the safety tests that selected bacteria are subjected to. These tests (Figure 3) are necessary to use these bacteria without any risk for humans, they begin as in vitro studies in laboratories and continue in animals and clinical trials in humans.
CONCLUSIONS
Understanding caries as an oral microbiota dysbiosis has raised again the issue of the development of strategies for the control of this disease. One of the biotechnological strategies is the use of probiotics, with the main objective of establishing beneficial microorganisms to contribute to homeostasis in the oral cavity. In oral health, the findings in a variety of studies on probiotics are promising, although few include clear results. Understanding the characteristics that probiotics should have for the oral cavity will allow selecting the most suitable strains for this purpose. This article discussed some of the most important features, prioritizing safety in their use and location in the oral cavity.
RECOMMENDATIONS
Probiotics could be used as a preventive treatment for tooth decay; however, in some countries like Colombia no products have been developed specifically for the control of this disease. This creates an invitation to conduct studies on bacteria that satisfy the ideal characteristics for the oral cavity and prove being effective.
Such studies could start by isolating and identifying native bacterial strains that are safe, in order to create a greater availability of options and select the most appropriate for clinical studies. If a strain with clear results is found, new products will be available in the market and probably public health measures as well.











 text in
text in