INTRODUCTION
Implants are an essential part in the field of restorative dentistry nowadays, and due to its proven success rate,1-5 they have largely replaced traditional treatments; however, to be successful, they need to comply with minimum conditions, the most important of which is the presence of a residual alveolar bone with proper thickness and height. For this reason, the practice of osseointegration has been expanded along with new complementary treatments to facilitate the fulfillment of this condition.6-11
Two key problems must be overcome in the posterior area of the maxilla: the pneumatization of the maxillary sinus and the reabsorption of the alveolar bone after extraction.7,12 These two phenomena combined lead to a quick and irreversible decrease in the available bone.
The maxillary sinus lifting technique was designed to improve this condition by lifting the sinus floor, increasing the height and amount of available bone, enabling the use of implants for the restoration of this area,11,13 with satisfactory results since its publication nearly two decades ago.13-15 However, the literature is not conclusive on the best material to use as a graft. The studies have reported the use of different materials, including autologous bone,11,15,16,17,18,19 homologous bone,20-24 xenografts,14,15,19,25 and alloplastic materials.1,14,25,26 Within these alternative, the autologous bone is the one with the closest to ideal conditions,2,27,28,29 but due to the disadvantage of a second surgical site donor and the morbidity this entails, the exploration of other alternatives was necessary.
Despite the progress in the search for new materials, the studies with bones other than the autologous ones have failed to overcome the advantages of the latter, so it becomes necessary to study all the possible donor sites, seeking an autologous bone with the best possible biomechanical properties and with minimum risks or post-surgery consequences.30,31,32
Concerning extraoral donor sites, the tibia provides several advantages over the iliac crest, which is currently the most commonly studied; these advantages include faster recovery time and a less traumatic, manageable postoperative period for the patient. In addition, it can be done under local anesthesia, without reducing the quantity or quality of available bone.32,33,34
The use of tibia bone has not been sufficiently documented. Additional studies are required to show that tibia grafts can provide conditions for optimal bone regeneration, with a manageable postoperative period for patients in terms of pain and inabilities.
The objective of this study is to compare the radiographic andhistologicalbehaviorofautologous bone extracted from tibia and demineralized freezedried homologous bone in the lifting of maxillary sinus, evaluating it in two ways: a) radiographically, by measuring the bone height obtained with both techniques before surgery, immediately after, and six months after surgery, and b) histologically, with bone samples obtained at the time of the insertion of the implant six months after surgery.
MATERIALS AND METHODS
This project was approved by the Research Center and the Ethics Committee of Universidad de Antioquia School of Dentistry. The study complies with the recommendations for biomedical research of the 1964 Declaration of Helsinki of the World Medical Association, as well as the scientific-technological and administrative standards for research in health, Resolution No. 008430 of 1993, issued by the Ministry of Health, in its title II, Chapter 1, on the ethical aspects of research in humans, articles 5, 8, and 11.
Between June 2007 and December 2009, 16 patients were surgically intervened in a random manner to perform maxillary sinus elevation before placing implants in the posterior maxillary region. 13 patients had the procedure done on one side of the maxilla and 3 had it on both sides, for a total of 19 operated maxillary sinuses. All patients had diagnosis of dental loss with alveolar ridge atrophy associated with maxillary sinus pneumatization. Cases with remnant alveolar ridge height below 6 mm in upper posterior areas were included, with no exclusion criteria in terms of minimum alveolar height. The following patients were excluded from the sample: smokers with a history of sinus pathology, patients with systemic diseases that could compromise the healing process or with not treatable severe sinus membrane rupture during the initial surgical procedure. Of the 16 patients, 11 were women and 5 men. The average age was 53 years, ranging from 23 to 66 years Table 1. In 10 maxillary sinuses, the procedure was done using freeze-dried homologous bone as graft material (Banco de huesos y tejidos, Fundación Cosme y Damián. Bogotá, Colombia) (Group 1), and autologous bone extracted from proximal tibia was used as graft in the 9 remaining cases (Group 2). All patients were operated by the same surgeon (JDM) using the technique described by Boyne and James (cited by Mish et al).8 Radiographic controls were conducted at three different times by means of panoramic radiography, using equipment of the Universidad de Antioquia School of Dentistry (Orthopantomograf OP100 from Instrumentarium Imaging). The first control was carried out prior to surgery (R1), the second one was made in the first week after sinus floor lifting surgery (R2) and the third was made 6 to 8 months later (R3). All patients were evaluated in the first two times, but at the end, for the third evaluation, 8 of them (4 of each group, all cases of unilateral elevation) did not continue the process (communication with 6 of them was not possible, and 2 left voluntarily), so the final number of evaluated sinuses reduced to 11 (5 patients with unilateral elevation and 3 with bilateral elevation).
Table 1 Histological evaluation. Samples 1 to 6 belong to Group 1; samples 7 to 11 belong to Group 2. The signs + and - mean presence or absence of each of the selected items
| Sample | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|
| 1 | + | - | + | + | + | + | - | - |
| 2 | + | + | + | + | + | + | + | - |
| 3 | + | + | + | + | - | - | - | - |
| 4 | - | - | - | - | + | - | - | + |
| 5 | + | + | + | - | + | + | - | + |
| 6 | + | - | + | + | + | - | - | - |
| 7 | + | - | + | + | + | - | - | - |
| 8 | + | - | + | - | + | - | - | - |
| 9 | + | - | + | + | + | + | + | + |
| 10 | + | - | - | + | - | + | - | - |
| 11 | - | - | + | - | + | - | - | - |
| Group 1 | 83.3% | 50% | 83.3% | 66.6% | 83.3% | 50% | 16.6% | 33.3% |
| Group 2 | 80% | 0 | 80% | 60% | 80% | 40% | 20% | 20% |
A: Amorphous eosinophilic material. B: Remnant grafted bone attached to the bone. C: Vital mature bone. D: Granulation tissue. E: Connective tissue. F: Blood vessels. G: Osteoblasts. H: Osteoid tissue. The percentage of samples showing each tissue type was calculated and is included at the bottom of the table.
Radiographic evaluation
Panoramic radiographs were used due to availability and low cost; in addition, a previous study by one of the authors35 showed that this type of radiograph is sensitive and specific enough to be used as a diagnostic tool in the assessment of sinus pathology, with a 95% confidence level compared to CT scans.
The radiographic analysis was performed by two evaluators at two different times, with the same results given the simplicity of the measurement.
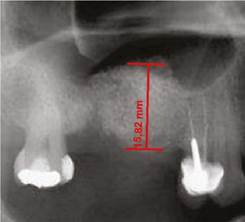
All images were digitized for the quantitative analysis of the area of interest using version 3.1 of the AxioVision software. The height of bone and graft was measured and adjusted according to magnification, using a 25% conversion factor. The initial measures were expressed in pixels and later converted to millimeters, using a millimeter ruler that was scanned with each radiograph. The measurements were taken from the highest point of the alveolar ridge in the region selected to place the implants during the next phase to the point with evidence of radiopacity change in an apical direction inside the maxillary sinus (Figures 1, 2, and 3).
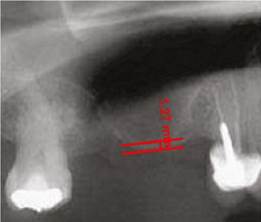
Figure 1 Residual height of the alveolar ridge in the posterior area of the maxillary before the sinus floor elevation procedure
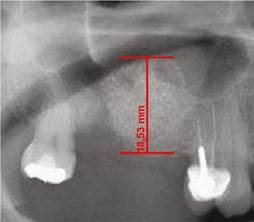
Figure 2 Alveolar ridge height immediately after the surgical procedure on the same patient inFigure 1. Ridge height increased by 17.2 mm.
Extraction of the tibia graft
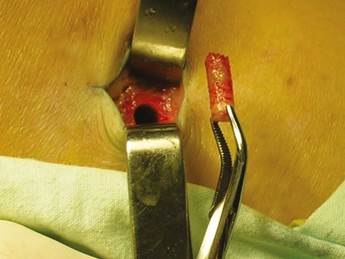
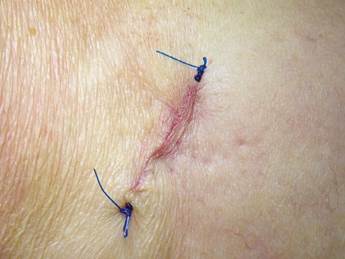
Patients were placed in supine position with partial knee flexion for better exposure of the tibia. After preparation of the entire surgical area with iodized soap, the sterile fields were located. The anatomical references were identified on the anterior surface of the proximal portion of the tibia. An oval protrusion called Gerdy’s tubercle can be felt between the condyles. Having this reference helps prevent complications involving surface joint of the knee and the head of the fibula, which is also located in this area. Under local anesthesia with two cartridges of the 2% lidocaine with epinephrine 1:80,000 (Roxicaina, Ropsohn Therapeutics. Colombia), an oblique incision of 2-3 cm was done with a #15 blade (Figure 4). The incision was deepened to compromise skin, subcutaneous tissue, the fascia of the iliotibial tract and the periosteum. The proximal portion of the tibia bone was obtained in its cortical surface by means of trephines of 5 mm in internal diameter by 14 mm in length (Trephine Meisinger T229L, Germany) and bone curettes in the deep area (Figures 5 and 6). A variable amount of medullary bone was extracted, ranging from 2-3 gr (5 cc). In the case of bilateral sinus lifting, the amount never exceeded 7 gr. The wound was closed by planes with vicryl 3-0 in the deep part and 6-0 nylon (Ethicon, Johnson & Johnson, USA) in the skin, and this was subdermal to achieve a more aesthetic appearance of the final scar (Figure 7). Drains were not used, and no attempts were made to fill the space on the bone epiphysis with any material.
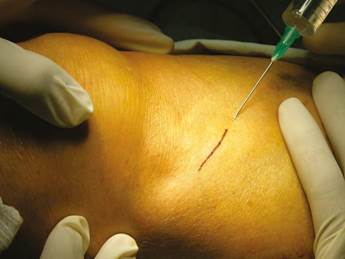
Figure 4 Design of the incision to reach the lateral proximal portion of the tibia prior infiltration of local anesthetic to extract autologous graft.
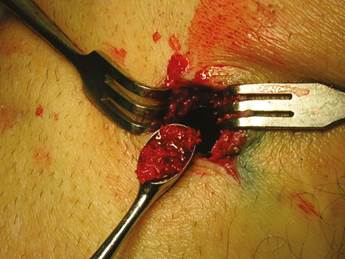
Figure 6 The medullary portion of the tibial epiphysis can also be collected with curettes when a greater amount of bone is required
Sinus lift
All patients were clinically and radiographically evaluated through panoramic radiography to establish the amount of bone atrophy and to discard the cases with obvious sinus pathology. All procedures were performed under local anesthesia with strict sterilization.
Local anesthetic was applied with vasoconstrictor, 2% lidocaine with epinephrine 1: 80,000 in the vestibular and palatal area. A crestal incision was done slightly towards palatal in all the edentulous maxillary ridges on the corresponding side. This was complemented with a relaxing vestibular incision in the anterior limit of the edentulous area, as well as a small distal releasing incision. A flap of total thickness was dissected to expose sufficient height of the lateral surface of the maxillary sinus to perform the procedure in a comfortable and safe manner. Taking into account the measures taken from the panoramic x-ray, the boundaries of the maxillary sinus were established. A rectangular or elliptical bone window with rounded corners was made using round low-speed carbide burs under continuous irrigation. Once the sinus membrane was exposed, the bone window was moved towards the interior of the sinus, with a rotating shaft in the upper horizontal osteotomy, avoiding harm to the sinus membrane.
Using blunt curettes of different angles (External sinus lift control. Meisinger, Germany), the membrane was separated from the bony walls of the maxillary sinus. In the event of rupture measuring less than 3 mm, a resorbable collagen membrane of 30 by 30 mm was used for immediate sealing (Genius, Baumer, Brazil) in order to block communication and prevent serious postoperative complications, continuing the procedure in a conventional way according to the protocol by Testori et al.36
Once the sinus membrane was lifted, the following types of bone graft were used: human demineralized freeze-dried bone in a group of patients, and autologous cortico-medullar tibia bone in a second group of patients. The graft material was first inserted in the posterior region, then medially and finally in the central area (Figure 8). The surgical wound was closed using vicryl Plus 4-0 suture (Ethicon, Johnson & Johnson, USA) by means of simple points in both the vertical incisions and the crestal area. Graft healing was allowed for a period of at least 6 months, and then the implants were placed in the area. During the implant procedure, the first bone preparation burs were replaced by a trephine of 2 mm in internal diameter and 3 mm in external diameter by 10 mm in length (trephine Meisinger T229, Germany), in order to extract a sample of bone tissue in cylindrical shape which would be histologically analyzed later. The preparation of the alveolus for the implant was made with conventional burs of the system selected in each patient, according to the treating prosthodontist, positioning implants measuring no less than 3.5 mm in diameter and no less than 10 mm in length, given the size of the bone defect created with the trephine.
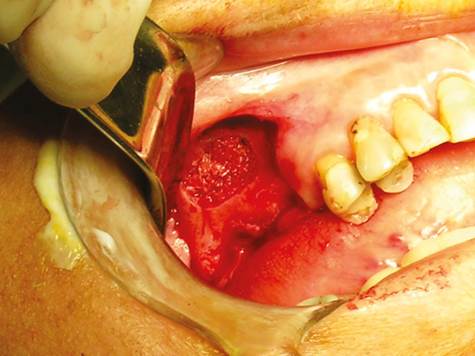
Figure 8 Aspect of the surgical window in the anterior wall of the maxillary sinus following maxillary sinus floor elevation and placement of the graft.
In both groups, analgesic therapy was prescribed according to each patient, as well as antibiotic therapy with Amoxidal duo cap 875 mg every 12 hours for 6 days (Roemmers, Uruguay). The intraoral and tibia skin suture points were removed at 15 postoperative days.
Processing of the samples for histological study
Sample processing was done at the Pathology Laboratory of the Universidad de Antioquia School of Dentistry by qualified staff and under the supervision of an oral pathologist.
The samples obtained during surgery were placed in individual bottles properly labeled and fixed on 10% formalin (pH 7.0) covering the entire specimen.
Each fragment was immersed in hydrochloric acid with polyvinylpyrrolidone (Shandon, TBD - 1, Rapid Decalcifier, Thermo Fisher Scientific. Kalamazoo, MI), according to the manufacturer’s instructions, for periods ranging from 2 to 16 hours. They were later immersed for 10 min in bicarbonate water to neutralize pH, and then embedded in cassettes for tissue processing (Histossetes, Simport. Canada), in order to initiate the process of fixation, dehydration, and clearance in the processor of tissues during 12 hours. Then they were immersed in liquid paraffin (Histoplast PE., Thermo Fisher Scientific) at temperatures ranging from 58 to 60 °C. Serial sections were then made to 5 µm using a microtome (Microtome M3500, M3500, Bright Instrument Co Ltd. Huntingdon, Cambridgeshire, England).
Three slides were obtained from each case and subjected to the following stains: a) hematoxylin and eosin, b) Masson’s trichrome stain, c) von Kossa stain. It is important to note that all slides were obtained from the deepest part of the bone cylinder, verifying patient’s bone height on the initial x-ray, taking the samples from a deep point to ensure that in all biopsies the obtained slides always corresponded to grafted bone instead of autologous bone prior to elevation.
Subsequently, an oral pathologist conducted the detailed morphometric analysis, using a light microscope, according to the instructions given by the researchers to verify the following conditions in each slide and stain:
H&E stain: presence or absence of amorphous eosinophilic material, mature vital bone, granulation tissue, connective tissue, blood vessels, osteoblasts, and osteoid.
Masson’s trichrome stain (modified from Goldner): percentage of osteoid (red stain) and mineralized bone (blue stain).
Von Kossa stain: Percentage of osteoid (pink stain) and mineralized bone (dark black or brown stain).
RESULTS
19 maxillary sinus floor lifting procedures were conducted on the 16 original patients using two different graft types. Group 1 was subjected to maxillary sinus elevation, complemented with freeze-dried homologous bone; four cases were performed on men and six on women. Group 2 patients were grafted with autologous tibia bone; two cases were performed on men and seven on women. The average age in Group 1 was 50 years (ranging from 23 to 57) and the average age in Group 2 was 58 years (ranging from 43 to 67).
In all patients from the second group, removal of the proximal tibia graft and its placement in the maxillary sinus was done in a single surgical session under strict local anesthesia in a period no longer than 20 min.
At the end, four patients from each group did not continue the process (all of them with unilateral elevation), so that the sample was reduced to 11 cases, six from Group 1 and five from Group 2.
As to complications, there was rupture of less than 3 mm of the membrane sinus in four patients, one from the freeze-dried bone group and three from the tibia group; however, the quick placement of bioabsorbable membrane allowed avoiding major complications, like sinusitis or graft resorption. In addition, one patient had a surgical wound infection on the area of extraction of the tibia graft, which was managed with antibiotic therapy with dicloxacillin cap 500 mg, four times a day for fifteen days (Diclocil, Bristol Myers Squibb, Colombia), and was resolved with no further complications. Minor complications in 5 patients of 16 shows a rate of 31.2%. However, it must be noted that 4 of 5 of these complications were minor sinus membrane perforation, which are related to the surgical technique and did not have a negative effect on the prognosis of the graft.
In applying statistical tests to the patients who completed the three evaluation periods, the Shapiro Wilk test showed, with 5% significance, that the variables were normally distributed. A t test showed that there were differences, with a 5% significance, between the averages in the three moments of evaluation (R1, R2 and R3) in both groups. An Anova test was applied to compare the results in the three evaluation times in both groups, finding out significant differences in Group 1 (5%, p > 0.05) in both at baseline and the radiographic evaluation immediately after the procedure, and between this second time and the evaluation 6 months afterwards. Significant differences were found in Group 2 between the preoperative radiographic evaluation and the one immediately after surgery, but there was no significant difference between the second time and the evaluation at 6 months. This means that there were significant differences in the initial alveolar height and that obtained with the surgery in both groups, as well as 6 months after the start of the healing process only in Group 1, but there were no major changes in bone height between R2 and R3 in Group 2, suggesting that there was a minimally significant post-surgical resorption specifically in patients treated with autologous tibia graft.
The initial height of the alveolar ridge in Group 1 patients was 2.9 mm on average (range 0,9-4,3 mm) and 3.9 mm in Group 2 (range 1,4-5,4 mm). Group 1 had an average initial height gain of 13.9 mm (range 9,9-17,7 mm) and in Group 2 the average gain was 10.3 mm (range 4,9-15,2 mm). Six months later, the cases in Group 1 showed an average loss of 2.9 mm (range 0,4-3,6mm) and only 1.9 mm (range 0,3-3,9 mm) in Group 2.
Histological findings: a total of 11 samples were processed, staining with hematoxylin and eosin, Masson’s trichrome stain and von Kossa stain to differentiate the osteoid from the mineralized bone.
The parameters described by Becker et al (1996) were used for each sample, finding out the results listed in table 1.
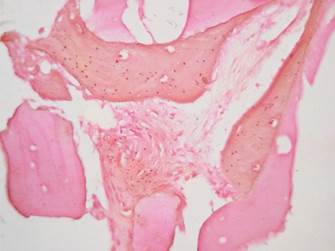
The samples from Group 1 and Group 2 had moderate amounts of amorphous eosinophilic material (Group 1: 83.3%, Group 2: 80%), mature vital bone (Group 1: 83.3%, Group 2: 80%), granulation tissue (Group 1: 66.6%, Group 2: 60%), blood vessels (Group 1: 50%, Group 2: 40%), osteoblasts (Group 1: 16.6%, Group 2: 20%) and osteoid (Group 1: 33.3%, Group 2: 20%). (Figures 9, 10 and 11).
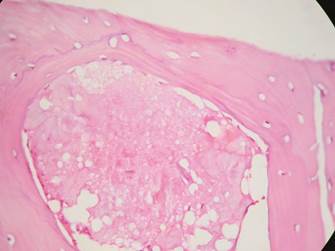
Figure 9 Group 1. H&E stain 40X. Amorphous eosinophilic material can be seen surrounded by mature bone
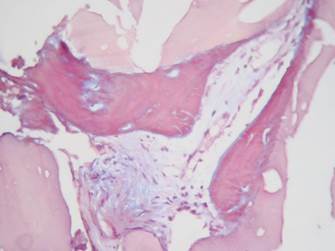
Figure 10 Masson’s trichrome stain (modified from Goldner) 40X. Group 2. Showing osteoid in red with small central islands of mineralized bone in blue.
DISCUSSION
This study compared the stability of tibia grafts and decalcified freeze-dried bone grafts in maxillary sinus floor lifting procedures prior to placement of implants. The results suggest that patients in the group grafted with freeze-dried bone showed significant differences between the height at baseline and the height found 6 months after the procedure. Whereas in the group grafted with autologous tibia bone, the initial height did not have a significant reduction during the 6 months following maxillary sinus floor elevation surgery (Figures 1 to 3).
These results are similar to those reported by the Consensus on Maxillary Sinus Floor Lifting in 1996,13 in which freeze-dried bone grafts showed an average height loss of 2.09 mm, compared to 2.9 mm in our study. The tibia grafts in our study had 1.9 mm decrease in height, comparable with the reduction shown by iliac crest grafting in the abovementioned report.
The histological findings of our study are similar to those reported in previous research with autologous grafts. Some authors report the production of lamellar and trabecular bone in moderate amounts, with small amounts of blood vessels and varying amounts of granulation tissue (Figure 9).11,29,37,38 The samples subjected to Masson’s trichrome stain and von Kossa stain showed similar results in both groups, suggesting the presence of new bone formation in both groups (Figures 10 and 11).
Regarding freeze-dried bone, there were similar results to the autologous bone, with moderate new bone formation. Another study reports similar findings,29 pointing out that the newly-formed bone is located in areas adjacent to the preexisting bone. This statement could not be confirmed or discarded in our sample.
In our study, the procedures of maxillary sinus floor lifting and implant were conducted in two phases. Even though there was a second surgical time, no major complications occurred. From the clinical point of view, the resistance shown by both the tibia bone and the DFDB at the time of implant placement and biopsy was similar to the type 3-4 bone in the classification by Lekholm and Zarb.39
Autologous bone is considered the gold standard among the different graft types, and it is the most predictableandmost successful alternativecurrently available.40,41 Availability is its main drawback. The need for a second surgical site has prompted the search for different substitute materials.42 DFDB is one of the alternatives to autologous bone; it is frequently used and has shown biocompatibility and osteoconductivity properties, as well as a slow reabsorption rate.23
Despite its limited availability, autologous bone is the graft material closest to the ideal conditions for reconstruction treatments.43 It meets requirements as important as biocompatibility and structural integrity.44 In addition, it stimulates the formation of new bone by osteogenesis, osteoinduction, and osteoconduction,20 it has an anatomical structure that allows cellular invasion while providing structural support to the receptor site, and is an important source of type I collagen, which facilitates the processes of vascularity and resilience, thus promoting osteoblastic activation.44
Autologous bone can be extracted from extraoral and intraoral donor sites. Intraoral donor sites include the symphysis,8 the mandibular ramus,2 the tuberosity,45 the coronoid process, and the zygomatic bone,46 and the extraoral sites include the cranial vault,47 the ribs, the anterior or posterior side of the iliac crest48,49 and the tibia.6,16,18 Site selection depends mainly on the amount of bone to extract and the treating surgeon’s preference.
The donor site determines the embryologic origin of the extracted bone. Endochondral bone grafts (tibia or iliac crest) show more reabsorption than bones of an intramembrane origin, in which even the final volume tends to increase.50 In our study, despite the endochondral origin of tibia bone, there was a low resorption rate and a bone of clinical characteristics suitable for an implant.
The extraction of autologous graft bone involves increased postoperative morbidity due to the intervention in a second surgical site, with increased risk of complications according to the chosen donor site.51 This is why the selection of an autologous graft donor site should seek a balance between the characteristics that will allow better graft performance and the least risk of complications or serious consequences for patients.
Proximal tibia bone is one of the donor sites that has been explored.6,16,30,34,51,52 Efforts to study this donor site were based on ease of access, the volume of available bone, and the little morbidity observed in a group of 206 orthopedic trauma patients reported by O’Keefe et al, who made 230 tibia bone grafts to manage fractures of the lower extremities, with excellent results.18
The tibia bone offers cancellous bone, 10 to 42 cc of medullary bone, with 28 cc on average,6,16,30,34,51,52,53,54,55 from the lateral or medial proximal area of the tibia through an incision of 2 to 3 cm in length.6 The extraction of more than 10 cc of tibia was not necessary in our study even in the bimaxillary elevation cases, keeping the structural integrity of the donor area.
This technique has advantages over other types of approaches used frequently, such as the iliac crest.32,34 These advantages include:
It is well tolerated by patients, using local anesthesia only;51,55 costs therefore decrease, since the procedure can be performed in a single session at the clinician’s office instead of an operating room, and it can be made entirely by the maxillofacial surgeon.
Postoperative morbidity is lower: after an extraction of iliac crest graft, the patient requires inability of 5 to 14 days,7,12,56 with difficulties to walk for up to two weeks, while with tibia bone extraction, the patient will be disabled for three days maximum, the expected post-operative period after extracting the graft is much more manageable, and there is only a slight alteration in patient’s mobility, which is recovered in its entirety two to five days after surgery,16,51 provided that the patient is warned against lifting heavy objects or performing sporting activities involving impacts to the legs, like playing soccer, for about three weeks, which is the minimum time required for proper filling of the medullar gaps in the tibial epiphysis.55
In addition, the risk of surgical complications is very low (1.7% minor complications), compared with the iliac crest surgery.56,57 In our sample, only one patient had an infection as a minor complication associated with the removal of the tibia graft, which was successfully managed with antibiotic therapy.
Allografts or homologous grafts21,40 come from individuals of the same species as the receiver and are divided into three main categories: deep-freeze, freeze-dried or lyophilized, and demineralized freeze-dried. They have been used since the 1970s because of their osteoconductive properties.22,58 In 1996 they were proven to meet the necessary criteria to induce periodontal regeneration.13 Their main advantage is that they can be used in the necessary quantities with no need for additional surgical sites.59,60 However, they lack osteogenesis properties, which is reflected in their histological behavior during the repair, characterized by particles surrounding the new bone when DFDB particles are close to native bone, but if they are far, they show little remineralization or limited formation of new bone.29 It has been suggested that, in combination with other biomaterials, DFCB can provide better results.58,61 Our sample of human DFDB showed varying degrees of bone formation which, due to the sample size, cannot be taken as conclusions clinically applicable.
Maxillary sinus lifting have been reported with other materials, such as alloplastic grafts,1,59,60,62,63,64 xenografts15,24,65 and bone morphogenetic protein (BMP).19,66,67,68 The use of this type of material has been extensively documented with mixed results.
Tibia bone offers advantages over other choices, such as the iliac crest bone and DFDB, specifically in procedures for maxillary sinus elevation. According to the results of our sample, the tibia bone shows better properties than the results obtained with DFDB and similar tissues in their healing patterns, osteogenic capacities, and long-term stability, compared with iliac crest bone, with evidence of less postoperative morbidity and better tolerance by patients; however, we cannot conclusively define if there are major histological differences between the two types of graft.
It should also be noted that the possible rejection of the autologous bone graft of tibia in some people, because of being an extraoral site, is highly compensated by the simplicity of its technique and ease extraction by trained hands; large quantities of bone can be obtained from this site without major complications for patients.
The versatility of intraoral donor sites is undeniable, but the use of proximal epiphysis of tibia for cancellous bone must be considered, especially in cases requiring large maxillary sinus lifting in combination with minimal bone height, for the placement of multiple osseointegrated implants.
Further studies are needed to directly compare the performance of tibia grafts against iliac crest grafts and the ones obtained from intraoral sites and other alternative sites, such homologous or heterologous freeze-dried bone, in order to confirm or dispute our findings. The results found in our sample are only applicable to it, and a sample with a greater number of patients is needed to provide results that can be applied to the general population.











 text in
text in