INTRODUCTION
In dentistry, asepsis is critical to avoid cross infections, post-treatment failures, unnecessary prescription of antibiotics, and complications that put patient’s life at risk.1,2,3 However, some clinicians and students still neglect this important concept.4,5,6,7,8 This is evident by the use of antibiotics as a preventive measure for inadequate asepsis techniques,9,10 and the absence of protocols for sterilization or disinfection of some materials to be used for the first time, as well as their improper handling before being used in dental procedures.8,11,12
During the endodontic therapy, clinicians seek to eliminate the microorganisms present in the root canal and to prevent the settlement of new microorganisms through the production of hostile conditions for such microorganisms 13,14,15 These conditions an be achieved by providing almost non-existent or insufficient amount of nutrients, limiting the space available to the microorganisms, altering the redox potentials, creating a low concentration of oxygen, and promoting the presence of antimicrobial substances.16 However, some studies show the presence of several microorganisms involved in infectious diseases that are able to adapt to these conditions,15,17,18 and thus validate the need to use materials and additions in conditions as aseptic as possible.
Among the microorganisms involved in endodontic failure and whose characteristics make them resistant to extreme conditions are Enterococcus faecalis, Staphylococcus aureus, and Candida albicans .18,19,20,21 These microorganisms are regarded as the toughest in the oral cavity.19,20,21,22E. faecalis is the microorganism most commonly found in high numbers in endodontic failure and usually enters the oral cavity during treatment.23,24 These bacteria are Gram-positive and facultative cocci that can survive in conditions that are commonly lethal for other microorganisms; theycan grow inlow-nutrient environments, have a high redox potential, are highly resistant under anaerobic conditions, can survive in a wide range of temperatures, pH, and salt concentrations, and are resistant to substances such as calcium hydroxide.18,19,20 In addition, they have the ability to invade the dentinal tubule, use collagen dentin, and form biofilm.21,25,26
Staphylococcus aureus is another bacterium also found in post-endodontic treatment lesions, although less frequently than E. faecalis. They are Gram-positive and facultative anaerobic cocci that can survive in harsh conditions for long periods and resist changes in temperature and dehydration. Most of these bacteria have been able to develop resistance to antimicrobial drugs.27,28
Candida albicans is a kind of yeast often isolated from infected root canals.18,19,20,21,22,23,24,25,26,27,28,29 This microorganism has similar characteristics to E. faecalis, such as survival in monoinfections and in environments with limited amount of nutrients, as well as extreme pH ranges. In addition, it invades the dentinal tubule, is resistant to several antimicrobial agents, and has the capacity to produce biofilms on different surfaces. This yeast also has collagenolytic enzymes that produce degradation of human dentin collagen.29,30
The goal of this study was to warn dental students and faculty members at the Universidad Cooperativa de Colombia at Villavicencio about the need to sterilize cast cores before being used in patients. The objective of the study was to identify and quantify the presence of E. faecalis, S. aureus, and C. albicans in cast cores manufactured for the university clinics during the first half of 2016.
MATERIALS AND METHODS
Descriptive study by convenience sampling, consisting of two phases:
Phase 1. Collection of cast cores
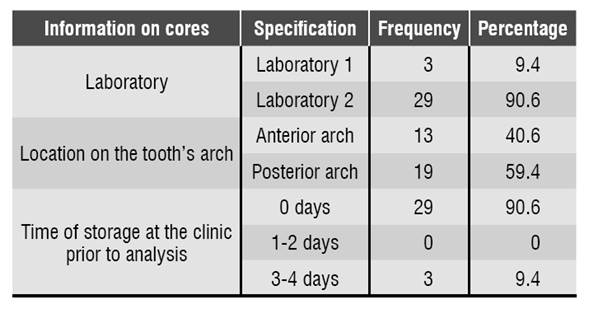
Manufactured cast cores were collected during the first half of 2016 at the clinics of Universidad Cooperativa de Colombia at Villavicencio. The study considered the ethical principles, requesting an informed consent from cast cores owners. 32 cast cores were collected provided that they met the inclusion criteria: manufactured cast metal cores -since this is the alloy most commonly used in this population- from patients who were provided an informed consent. The researchers collected information on the cast cores, such as manufacturing date, dental laboratory, location on the tooth’s arch and storage time before the analysis, which was recorded on a form assessed by an endodontist and a microbiologist.
Phase 2. Microbiological analysis
Media and growth conditions
The cast cores were placed in sterile conditions in microtubes containing 1 ml of saline solution.31 The microtubes were manually stirred for 30 to 40 s. From this tube, dilutions were made up to 10-4 and 100 µl of dilutions were sown in duplicate, in differential media for the studied microorganisms. CHROMAgar UTI 32 was used for E. faecalis, Baird Parker 33 was used for S. aureus, and Chrome Candida for C. albicans.34 These media were purchased from a certified company that distributes media with quality control using reference strains. Once the culture was completed, the media were incubated at 35 °C for 24 to 48 hours. Later the cast cores were brought back to the dental clinic, to complete the sterilization process before being cemented in patients.
Identification and confirmation of microorganisms
To identify the microorganisms, the researchers selected and described the colonies showing the color and characteristics of the microorganisms in the used media Figure 1. The blue colonies were chosen in the case of CHROMAgar UTI, the black colonies were selected for the Baird Parker medium, and the green ones for the Chromo Candida medium. The colonies’ cells’ morphology was observed on the microscope by Gram staining. In the CHROMAgar UTI, colonies showing Gram- positive cocci were subjected to the catalase test, searching for negative catalase microorganisms as identifiable candidates Figure 1. In the Baird Parker medium, the black colonies showing Gram- positive cocci were subjected to the catalase and coagulase tests, even if they did not show a halo around them, seeking positive catalase and coagulase colonies Figure 1.
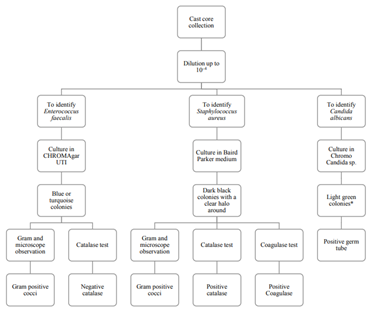
* No green stained colonies were observed in this medium
Figure 1 Procedure for the detection of Enterococcus faecalis, Staphylococcus aureus, and Candida albicans in cast cores. Source: The au- thors’ own elaboration.
Once the colonies of interest were selected, they were brought to blood agar and sent to a certified laboratory to confirm their identification. The same was done with some of the microorganisms that were observed more frequently. The identification technique used in the laboratory was the automated VITEK system.35
Quantification of colonies
Colonies showing the expected stain in the differential medium were quantified, as well as those identified as positive for the studied microorganisms. We calculated the number of CFU/ml of each cast core, by first calculating the average between copies and then transforming the quantification from microliters to milliliters.
Statistical analysis
Univariate descriptive analyses were conducted using the SPSS 20.0 software, including mean and standard deviation of the colonies in the media.
RESULTS
90.6% (n = 29) of the cores analyzed in this study were manufactured in dental laboratory N.° 2. Regarding the position in the dental arch, 59.4% (n = 19) were located in the posterior arch and 40.6% (n = 13) in the anterior arch. 90.6% (n = 29) of cores were analyzed in the laboratory immediately after being brought to the clinic, and 9.4% (n = 3) were stored for 3 to 4 days Table 1.
Microbiological analysis of E. faecalis, S. aureus, and C. albicans
The use of CHROMAgar UTI, Baird Parker, and Chromo Candida allowed the differentiation of colonies as well as a more selective isolation of the studied microorganisms. The average possible colony for E. faecalis in medium CHROMAgar UTI was 3,7x104 ± 1,5x105 and for S. aureus in medium Baird Parker was 1,7x104 ± 7,8x103 Table 2. No C. albicans colony was observed.
Table 2 Presence of candidate colonies in the differential media
| Medium | Average (CFU/ml) | Standard Deviation |
| Blue colonies in CHROMAgar UTI medium | 3,7x104 | 1,5x105 |
| Black colonies in Baird Parker medium | 1,7x104 | 7,8x103 |
| Green colonies in Chromo Candida medium | 0 | 0 |
Cores N° 2, 16, 17, 26, 27, 28, 30 and 32 had a greater number of possible colonies for E. faecalisFigure 2. Gram-negative (short and long) bacilli were predominant among the colonies, followed by Gram-positive cocci Figure 3. The catalase test applied to colonies presenting Gram-positive cocci showed that the ones corresponding to core #26 was catalase (-) and was identified as E. faecalis. The quantity of this bacterium was 5x104 CFU/ml Table 3. This core was manufactured in Laboratory 2, was located in the posterior arch tooth and was not stored before analysis.
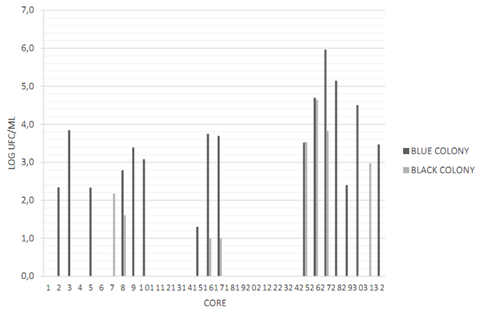
Figure 2 Presence and quantification of possible candidate colonies for the studied microorganisms. Data transformed to log CFU/ml. CHRO- MAgar UTI medium (blue stained colony) and Baird Parker medium (black stained colony). Source: the author’s own elaboration.
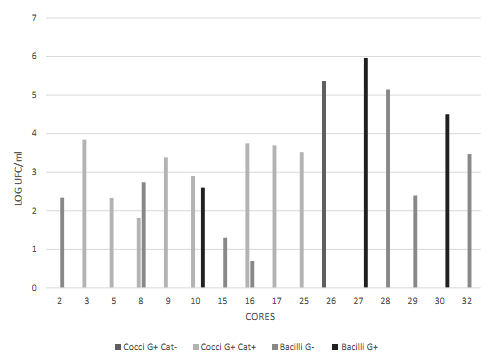
Figure 3 Cell morphology of the blue colonies present in the CHROMAgar UTI medium. Data transformed to log CFU/ml. Observed bacilli showed different characteristics (long, short, with and without spores). Source: the author’s own elaboration.
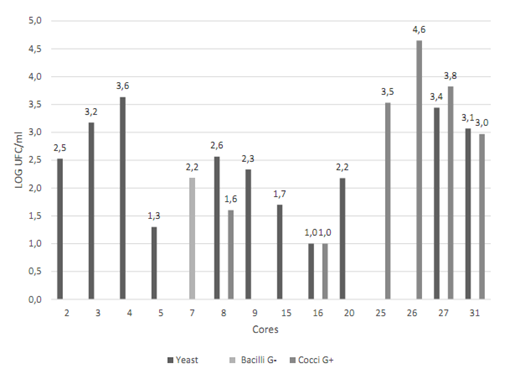
Figure 4 Cell morphology of the colonies present in the Baird Parker medium. Data transformed to log CFU/ml. Source: the author’s own elaboration.
Cores N° 7, 25, 26, 27, and 31 had a greater number of black stained colonies Figure 2, but with no halo formation. To ensure the absence of S. aureus, Gram staining, catalase and coagulase tests were conducted, with the following results: Gram-positive cocci, positive catalase and negative coagulase Figure 4, which allowed discarding colonies of S. aureus.
Other identified microorganisms
In the CHROMAgar UTI medium, in addition to E. faecalis, Kokuria kristinae was identified in 4 cores (16.1%) with an average concentration of 3.6x102 CFU/ml ± 1.3x103, and Stenotrophomona maltophilia in one core (3.2%) with a concentration of 4.4x102 CFU/ml. In the Baird Parker medium, S. saprophyticus was detected in 4 cores (12.9%) with an average concentration of 1.7x103 CFU/ml ± 7x103, and the yeast Candida parapsilosis in 11 cores (35.5%) with an average of 3.4x102 ± 9x102 Table 3.
Table 3 Presence of E. faecalis, S. aureus, C. albicans, and other micro-organisms in the cores manufactured for the UCC dental clinic at Villavicencio
| Microorganism | Frequency of microorganism | Amount of microorganism |
|---|---|---|
| E. faecalis | 1 (3.2%) | 5x104 CFU/ml |
| S. aureus | 0 | 0 |
| C. albicans | 0 | 0 |
| Other identified microorganisms | ||
| S. saprophyticus | 4 (12.9%) | Average: 1.7x103 CFU/ml ± 7x103 |
| C. parapsilopsis | 11 (35.5%) | Average: 3.4x102 ± 9x102 |
| C. tropicalis | 2 (6.5%) | Average: 3x101 CFU/ml ± 1x102 |
| K kristinae | 5 (16.1%) | Average: 3.6x102 CFU/ml ± 1.3 x103 |
| S. maltophilia | 1 (3.2%) | 4,4x102 CFU/ml |
Source: the author’s own elaboration. ± Standard deviation
Regarding the medium to quantify C. albicans, none of the cores presented the formation of green stained colonies, as noted above. However, two cores had dark blue colonies (6.5%), which were identified as Candida tropicalis with an average concentration of 3x101 CFU/ml ± 1x102 Table 3.
DISCUSSION
Several studies have shown that the microbiota present in endodontic-treated root canals differs from the one normally found in untreated teeth.36,37 This suggests the important role of external microorganisms in endodontic treatment failure and therefore the importance of maintaining an asepsis protocol during all treatment steps.38 Otherwise, microorganisms and their derivatives can be transferred o the interior of the canal, reaching the apical area and even the alveolar bone.2,38
Although microorganisms are expected in cast cores after manufacturing, it became necessary to identify the presence of microorganisms that can cause endodontic failure, in order to show that this type of additives may bring microorganisms capable of producing post-treatment infections, and to educate the academic community on the need for sterilization prior to use on patients.
In this study, as in the one conducted by Ensinas et al,39E. faecalis was found in cast cores. Although the natural habitat of this microorganism is the gastrointestinal tract, it can also be found in the environment, namely in water, soil, manufactured materials, or fermented products, due to its great resistance to extreme conditions.40 It is therefore normal to find this bacterium in cast cores, but if they are not properly sterilized they can be transferred to the treated root canal causing infection.20,24
E. faecalis was quantified in 5x104 CFU/ml, a high concentration that can cause infection. In a study conducted by Melo et al, the authors established that a concentration of 101 and 102 UFC of E. faecalis, in root canals of gnotobiotic mice was sufficient for the settlement of the bacterium in 83.33% of cases.41 In addition, the literature reports that just a few viable cells of microorganisms capable of settling after endodontic treatment is sufficient to produce failure.42
While S. aureus and C. albicans were not identified in the cast cores analyzed, Ensinas et al reported them in their microbiological analysis.39 This may be due to the fact that the materials analyzed in this study were not in contact with S. aureus and C. albicans, or other types of microorganisms could cause their inhibition.43,44 Another reason has to do with the used methodology, since Ensinas et al placed the cores in an enrichment medium before immersing them in common media, thus increasing the concentration of cells of these microorganisms.
This means that, if there is only one cell in the cores, by placing them in the enrichment medium their concentration increases and therefore their identification is more likely. In the present study, in order to determine the quantification of the analyzed microorganisms, dilutions and cultures were performed in the differential media with no prior enrichment.
The other microorganisms identified in the cores in this study have also been implicated in endodontic failure or other infections. This study identified two yeasts of the Candida genus. This includes C. tropicalis, which after C. albicans, C. glabrata, and C. krusei is the yeast most frequently isolated in infected root canals.45 This yeast causes infections globally, is the most prevalent after C. albicans, and shares many pathogenic traits with this yeast, as the capacity to form biofilms and the resistance to some antifungals such as fluconazole, as well as to calcium hydroxide. In addition, C. tropicalis has been associated with fungaemia and meningitis.46,47,48,49,50
Another yeast identified in this study is Candida parapsilopsis, which is among the three most frequent etiologic agents of candidiasis worldwide. In Latin America, the frequency of invasive candidiasis because of this yeast has increased recently.50 Just as C. albicans, C. parapsilopsis has the ability to form biofilm, grow in environments with low oxygen content, and show resistance to calcium hydroxide, chlorhexidine gluconate, and fluconazole.50,51 In addition, it has been isolated in infected root canals and in acute apical periodontitis.49,52,53
A bacterium found in this study was K. kristinae, which is known to cause bacteremia associated with the use of catheters and with infective endocarditis. It has also been identified in samples of acute dental infection in pediatric population.54 Another bacteria identified was Stenotrophomona maltophilia, which is considered an opportunistic bacterium capable of producing septicemia, endocarditis, meningitis, bacteremia, and abscesses, among other complications. This bacterium has intrinsic resistance to β-lactam antimicrobials.55 Finally, S. saprophyticus was also identified; this microorganism produces urinary tract infections, but it has also been linked to bacteremia.56
Regarding the methodology used in this study, the process of analysis was streamlined for the presence of E. faecalis, S. aureus, and C. albicans in dental materials. The used media allowed the differentiation of colonies and the subsequent identification of one of the studied microorganisms by means of simple inexpensive tests, such as Gram-staining, catalase and coagulase.
CONCLUSIONS
This study identified E. faecalis in one of the cast cores (3.2%) manufactured for the dental clinic of Universidad Cooperativa de Colombia at Villavicencio. While S. aureus and C. albicans were not identified, other microorganisms that can cause endodontic infections or other complications were found, such as C. parapsilopsis (35.5%), C. tropicalis (6.5%), K. kristinae (16.1%), S. saprophyticus (12.9%), and S. maltophilia (3.2%). Therefore, it is extremely important to establish protocols for the sterilization of these materials prior to use.
RECOMMENDATIONS
It is necessary to optimize procedures in dentistry, not only to secure treatment success, but also to reduce infections that may affect the health and life of patient. In addition, it is important to bear in mind that the strict establishment of asepsis protocols during all the steps of the endodontic treatment will also help reduce the prescription of antibiotics or antifungals. Finally, it is recommended to use the methodology conducted in this study for the analysis of this type of microorganisms in dental materials or other type of materials.











 text in
text in