INTRODUCTION
The endodontic treatment aims to eliminate microorganisms from the root canal system and focuses on preventing the introduction of new pathogens in it. These microorganisms can originate from primary infection, or they can be introduced during endodontic manipulations. Studies indicate that microorganisms and their products are associated with endodontic treatment failure and perpetuation of periapical diseases,1,2 being considered as primary etiological agents of pulpal necrosis and apical periodontitis.3,4 Although non-microbial factors can contribute to endodontic failure, scientific evidences report that persistent infections within root canal, or secondary infections are the principal causes of endodontic failure.2,5 Attention should therefore be paid to the presence of microorganisms in the dental structure: pulp chamber, root canal system, dentinal tubules, as well as metabolic end products of bacteria with potential antigenic action.
When complete root canal disinfection is achieved by chemical and mechanical preparation, maintaining this disinfection is necessary. In the first instance, introduction of new microorganisms must be avoided during the root canal filling procedures, as their elimination is important. Consequently, it is of utmost importance that instruments or materials introduced within the root canal system do not contribute to the reinfection, or even to the persistence of the endodontic pathology.6 Thus, gutta-percha cones must be free from microbial contamination at the moment of use.
The scientific literature indicates that gutta-percha cones taken from manufacturer boxes before the first use don’t need to be sterilized. Contamination occurs mainly with continued handling of the boxes, and it may happen by exposure to the physical environment, inappropriate handling by the endodontic professional, or accidental contamination.7,8,9
Endodontic studies recommend that the guttapercha cones should be decontaminated before being placed within root canals. However, the incidence of contamination is still a reason of disagreement.7,9,10
There are plenty of studies evaluating the contamination of gutta-percha cones prior to first usage or the disinfection protocols; however, studies evaluating this occurrence in dental clinics are still lacking. Therefore, the objective of this study was to evaluate the occurrence of contamination of gutta-percha cones stored in manufacturer boxes already manipulated by clinicians and specialists.
METHODS
Gutta-percha cones of medium size from different commercial brands were evaluated. Thirty boxes in clinical use were collected: fifteen from general practitioners offices, and fifteen from endodontic specialist’s offices. The boxes were closed and inserted in previously sterilized surgical-grade paper with film Figure 1A). The packages remained sealed until the tests. The entire experiment was conducted under aseptic conditions.
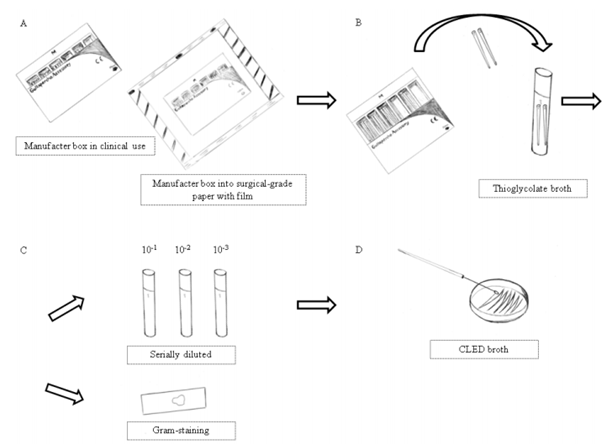
Figure 1 Diagram of materials and methods. Procedures: (A) manufacturer box in clinical use inside surgical-grade paper; (B) gutta-percha cones transferred to thioglycolate broth; (C) Fractions of solutions with turbidity were serially diluted, Gram-stained; (D) and plated in CLED agar.
In the Microbiology Laboratory, new procedures were performed inside a laminar flow cabinet Bio Protector 09 (Veco, Campinas, SP, Brazil), previously decontaminated with ethanol 70% and sterilized using ultraviolet light for 15 min; the operator used surgical gloves and sterile instruments. Two cones were removed from each of the 30 boxes using cotton pliers and transferred immediately to a tube containing 15 ml of fluid thioglycolate medium (fluid thioglycolate medium, Merck, Darmstadt, Germany) Figure 1B). The gutta-percha boxes presented some divisions, and the cones were removed from the emptier section(s). A new flamed plier was used for each box of cone. For each box, the above cited procedures were performed in triplicate totaling 180 medium cones and 90 tubes/tests Figure 1C).
The tubes were incubated for 21 days at 37 ºC, in aerobiosis, and analyzed daily for occurrence of turbidity. The tubes that presented turbidity at visual inspection were vortexed for 30 s. The solutions were submitted to sterile saline solution tenfold diluted up to 10-³. For the selective bacteriological identification at qualitative evaluation, aliquots of 0,1 ml of those solutions were plated in Cystine-Lactose-Eletrolyte-Deficient (CLED) agar (Merck, Darmstadt, Germany). The seeding was conducted by staging with sterile suspender platinic. The agar plates were incubated at 37 ºC under aerobic conditions and evaluated after 24 h Figure 1D). An aliquot from each thioglycolate broth that presented turbidity was subjected to Gram staining. A Nikon ECLIPSE E200 microscope was used to evaluate the plates.
To separate the control tubes (non-contaminated) that showed turbidity, the test was carried out in duplicate using the medium thioglycolate at the same incubation conditions of temperature and aerobiosis described previously for 48 hours.
A statistical analysis was performed to verify the difference between general practitioners and specialists regarding the presence or absence of contamination. The Chi-square test was used. The significant level was set at 5%.
Controls
One tube containing the culture medium, but no sample, was used as negative control of thioglycolate medium, and one CLED agar plate with no inoculate cone was used for the same purpose. Two cones removed from a new package (Dentsply, Brazil) and intentionally contaminated with Staphylococcus aureus (ATCC29213) for 24 h in trypticase soy broth were used as positive control. This positive control was made in triplicate. The solution with Staphylococcus aureus (ATCC29213) was plated onto a CLED agar plate as positive control. All tubes and plates were incubated in the same conditions as described above.
RESULTS
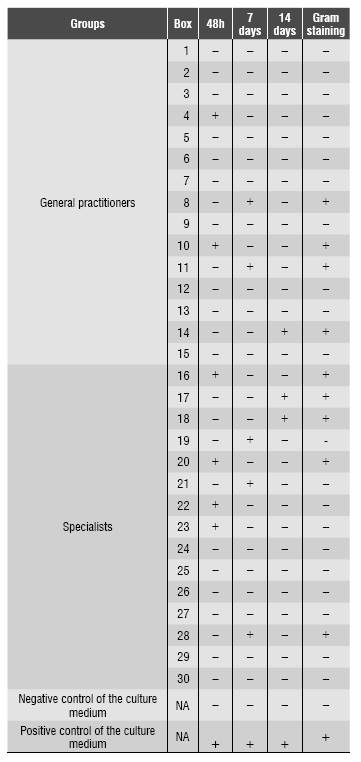
From 30 evaluated boxes, the thioglycolate tubes of 14 boxes showed modified optical density (turbidity) at different times: six samples after 48 hours; 5 samples after seven days and 3 samples after 21 days. Negative and positive controls yielded the results expected. From samples that showed turbidity in thioglycolate, 9 boxes (9/30=30%) showed bacterial contamination in Gram staining, 4 samples (4/15=13%) showed bacterial contamination, and 5 samples (5/30) did not. Four boxes (4/15=13%) came from clinicians and five (5/15=17%) from specialist’s boxes (16,6%). There was no significant difference in the level of contamination of cones in relation to their origin (p>0,05). Gram-negative and Gram-positive rods, as well as Gram-negative cocci and fungi were observed Table 1. In agar CLED, two plates showed bacterial growth confirming the presence of Staphylococcus aureus in two boxes.
DISCUSSION
The development or persistence of periapical diseases after endodontic procedures is mainly related to the presence of bacteria inside the root canal system.2,5,11 Efforts must be made to completely remove microorganisms from root canals,9,10,11,12 and to prevent introduction of others inside the system during the endodontic treatment or afterwards.
Studies of bacterial culture where quantitative data were evaluated to determine the connection between persistent microorganisms and endodontic treatment results have shown that the occurrence of positive culture is a cause for bad prognosis.2,3,4 Thus, the professionals must establish effective therapeutic protocols to eliminate microorganisms, with rigorous maintenance of aseptic conditions to prevent secondary infection during treatment.5,10,11,13
Since its introduction in endodontics, gutta-percha has been considered the ideal material as root canal sealer.14 This material presents percentages of some compounds that vary according to the manufacturer;15,16 however all the different brands include a component that shows antimicrobial activity: zinc oxide.17
To expand the antibacterial properties of gutta-percha cones, the addition of several components has been proposed in their formulation, including polyvinilpyrrolidone-iodine,18,19 calcium hydroxide (CA(OH)2 ,19,20 chlorhexidine (CHX),19,20 and iodoform.21 The antibacterial activity of cones, when associated with other substances, such as CHX, Ca(OH)2 or iodoform, did not provide advantages over the usual cones.19
Gutta-percha cones, even taken directly from unopened packages, become potentially contaminated after first clinical contact with the environment. The studies by Higgins21 et al demonstrated that the risk of contamination on gutta-percha cones immediately upon removal from the package was not a concern. However, Namazikhah et al22 concluded that, if the gutta-percha wasn’t intentionallycontaminated, thereis no need for decontamination prior to root canal filling.
Several studies attributed the microbial colonization difficulty of gutta-percha cones to the antimicrobial property exhibited by the zinc oxide presence in their composition;6,17 furthermore, the endodontic sealers also have antimicrobial activity. Other studies evidenced bacterial growth in samples of cones removed from unopened boxes.7,9 These conclusions should be carefully analyzed. It must be considered that, when the gutta-percha cone is coated with the sealer, some areas may remain uncovered during the insertion into the canal, or even during the overlay of cones by sealer. Justifying the presence of voids in root canal fillings without a sealer has frequently been pointed out by the literature, and this is a big problem.23,24,25
Da Motta et al.25 claim that the simple environmental exposure of cones is not of critical importance. However, we emphasize that the basic principle of infection control should be respected, and different studies evidenced the microbial contamination during frequent handling of gutta-percha packages.7,9,18
The use of endodontic material inappropriately sterilized will induce therapy failure, increasing the risks of introduction of microorganisms within the root canals systems.6,26 Incontestably, the operator shall rigorously maintain biosecurity by preventing the contamination of the sealing instruments and materials. Several studies emphasize the need of decontamination of gutta-percha cones immediately before their use.7,9,10,27 Some gutta-percha disinfection protocols can be performed, such as cone immersion in 5,25% NaOCl for 1 minute,6 use of 2.5% NaOCl or 2% chlorhexidine solutions for the same period of time,28, 29 or 1% peracetic acid for 1 minute for rapid disinfection.30
In the present study, we could observe contamination in 30% (14/30) of the boxes of evaluated gutta-percha cones, already in clinical use. The percentage of 13,3% (4/15) corresponded to samples from a clinicians and 16,6% (9/15) corresponding to specimens provided by endodontic specialists. Pang et al.29 found a comparable contamination percentage (19.4%) in a similar study. The authors assessed the contamination and disinfection of 150 gutta-percha cones from endodontic clinics divided into two groups. The first group was evaluated by measuring turbidity after immersion in 5.25% NaOCl, 2% CHX, and ChloraPrep (Medi-flex, KS) for 1, 5, 10, or 30 minutes and drying; and the second group was evaluated by the polymerase chain reaction method. Their results indicated that 19.4% of gutta-percha cones from the clinic were contaminated and all the species were Staphylococcus spp. Regarding the disinfection protocol, the three chemical disinfectants were effective in the rapid disinfection against Staphylococcus spp, and 1-minute immersion of the gutta-percha cones was adequate.
Contamination was expected to be higher among specialists because their boxes suffer greater use due to the large number of root canal treatments compared to those carried out by clinicians; therefore, the packages are exposed continuously during clinical use andmay be subjected toadditional contamination. However, this hypothesis was not confirmed, since the statistical analysis showed that the difference was not significant. Probably, a more rigorous training of these professionals makes them more concerned and more aware of biosecurity, balancing the contamination and the frequent handling of gutta-percha packages. Another probable reason is that, due to a greater number of treatments, the cones of boxes are exhausted more rapidly, making them less susceptible to environmental contamination. Another hypothesis is that general practitioners carry out less endodontic treatments during their routine, and therefore their boxes can be more susceptible to contamination due to the large environmental exposure. Consequently, the more the exposure the higher the contamination would be.
Concerning the detection techniques of microorganisms used in the present study, the main advantage of technical cultivation is the nature of the broad spectrum that it covers, which can identify a wide variety of species in the sample, even though it requires long periods for the assessment and identification of fastidious and demanding bacteria.31
The thioglycolate broth was the principal enrichment medium used in the experiment, providing adequate nutrients for growth of microorganisms usually present in low numbers, or slow growth, as well as fastidious and demanding ones. In tubes where the thioglycolate medium showed a change of optical density detected visually, the contamination was confirmed with the Gram method. The rebound indicated that turbidity possibly originated from any released organic dye used by different manufacturers, as reported in the literature.32 This explains the fact that some samples did not show contamination but produced turbidity of the medium. Only two samples showed growth on CLED agar, which is a culture medium for differentiation, isolation, and enumeration of bacteria in urine. This medium was originally developed to support the growth of pathogenic agents and urinary contaminants; also, it was used due to the ease of identification of important pathogens associated with cross-infections, being suitable for isolation of many microorganisms in aerobic growth, although a differentiation can be done according to the fermentation of lactose and some diagnostic tests directly in this medium.
The microbial contamination observed in the present tests finds support among many studies, which also yielded the need for decontamination of gutta-percha immediately preceding the filling of root canals, especially studies investigating the presence of microbial contamination of gutta-percha in unopened packages,9,23,30,32 and other studies evaluating this contamination in packages already in clinical use.23,30
Proper sterilization of gutta-percha cones can be considered difficult or impossible to achieve during daily clinic practice. However, several studies available in the literature demonstrate the laboratorial effectiveness of disinfection cones procedures, such as immersion for 1 minute in 5,25% NaOCl6; 2,5% NaOCl or 2% chlorhexidine solutions,8,29 or 1% peracetic acid for rapid disinfection.30
CONCLUSION
Our study demonstrates that bacterial contamination of gutta-percha cones in boxes for clinical use is frequent and was not different between general practice clinicians and endodontic specialists. These results can even emphasize the importance of implementing a strict disinfection protocol before using gutta-percha cones, due to the frequency of contamination.











 text in
text in