INTRODUCTION
The use of fluorides has proven to have a positive effect on the prevention of tooth decay and has been considered one of the most important public health measures of the 20* century.1,2 Its administration (which can be topical or systemic) aims to maintain a constant concentration of the fluoride ion (F-) in the oral cavity to facilitate the incorporation of these crystals on the surface of the erupted enamel-decreasing demineralization rate and increasing remineralization rate-.3 However, currently it is known that the excessive ingestion of F- has deleterious effects on enamel development, generating a hypomineralized porous phenotype with reduced hardness.4 In addition to the aesthetic and functional consequences, an in-vitro study conducted at Unidad de Investigación en Caries (UNICA) in extracted teeth with moderate fluorosis from Colombian patients, suggests that the porosity of the enamel with fluorosis makes it more susceptible to demineralization.5
Unlike the etiologic factor of dental fluorosis -which is fully identified as the chronic exposure to high concentrations of F- between the ages of 0 and 5 years-,6 little is known about the cellular and molecular mechanisms affected by F- and leading to the development of fluorosis. In Colombia, there are epidemiological reports7 that identify the sources of intake of F- possibly responsible for the high prevalence,8,9 but the pathogenesis of the defect has not been properly investigated.
We know that the chronic and sustained presence of the F- ion in plasma increases the likelihood of adhering to tissues in the mineralization process,10 but there is the misperception that the hypomineralization observed in dental fluorosis is the only consequence of the excessive addition of F- in enamel. In this literature review, we assume that the hypomineralized phenotype of fluorosis is the outcome of a series of possible effects of the F- ion on cell physiology and the proteins responsible for guiding the mineralization of enamel (biomineralization). First, we will make a brief introduction to the normal process of enamel biomineralization, and then we will study the available evidence on the effects of F- during key moments of the process.
Amelogenesis and enamel biomineralization
Enamel is a nano-compound bioceramics11 with 95% inorganic material, 4% water, and 1% organic matter.12,13 Four elements are required for the formation of enamel: cell, ions, proteins, and a compartment where the mineralization reaction takes place (extracellular matrix).12 The entire process of enamel formation is called amelogenesis, while the mineralization process as such, which takes place between ions and proteins secreted by cells, is called biomineralization. The enamelforming cells are called ameloblasts, and during the process they go through a series of changes that are summarized in the following stages: differentiation, secretion, maturation, and transition14 During the phase of secretion of ameloblasts, they carry ions from the plasma into the interior of the cell: the type of ion that enters will depend, among other factors, on its availability in the plasma at the moment.15 This is why the mineral of enamel (which is similar to the pure mineral hydroxyapatite) usually contains a variety of ions, such as HPO 42-, CO3 2-, Na+ y F-.16
In addition to transporting and secreting ions, ameloblasts synthesize and secrete a large amount of proteins, which are the major component of enamel in formation (> 90%).17 Among the secreted proteins are first those of the extracellular matrix (amelogenin, ameloblastin, and enamelin) and secondly the proteases: metalloproteinase matrix 20 (MMP-20) and kallikrein 4 (KLK-4). The enamelin andtheameloblastinfunctionasnucleators,attracting ions to their protein structure to favor the organized deposit of crystals of calcium phosphate salts.18
On the other hand, the amelogenin takes a higher supramolecular organization (20 nm nanospheres) and works as a “scaffold” that guides the growth of crystals for the formation of prisms.19 The role of ameloblastin is less clear, but it has been found to have functions in the adhesion and control of ameloblasts differentiation.20 The MMP-20 gradually and selectively degrades the protein support during the secretion and maturation stages, to allow the widening of the enamel crystals which previously grew in length.21 At the start of the maturation stage, cells stop producing MMP-20 and begin to produce KLK-4, a protease that completes the process of degradation of enamel protein material.22 The KLK-4 cuts the remains of structural proteins into small peptides that can be processed in the cell.23 At the end of this orderly process of the maturation stage, the protein component will be less than 1%11 and the resulting enamel will have minimum porosity and a translucent shiny look with a smooth feel.
Based on the evidence about the normal mechanisms of enamel formation (summarized above), several studies on the induction of fluorosis have been conducted to understand which steps of the process are affected by fluoride, as described below.
What concentrations of fluoride are used in in vivo and in vitro experiments for the induction of dental fluorosis?
Despite the existence of standardized models (described below), there is no consensus as to the concentration of F- biologically relevant to induce dental fluorosis in in vivo and in vitro studies. On the one hand, a line of evidence in the studies uses micromolar (µM) concentrations of F- and considers that concentrations of 2-12 µmol/L are biologically relevant.24,25,26,27 Another line of evidence argues that, under normal conditions, there are basal levels of F- in the fluid of enamel which proportionally increase in the presence of concentrations of F- in plasma and expose the ameloblasts to milimolar concentrations (mM) of F-.28 The difficulties for a consensus and the directly proportional relationship between the dose of F- and cellular responses make it necessary to examine the effects reported for F- along with the concentration used in the experiments.
In vivo and in vitro models standardized for the study of dental fluorosis
Two in vitro models have been established: the first one consists of a line of immortalized cells similar to ameloblasts, resulting from the enamel organ of the first molar in newborn Swiss-Webster mice. This line was achieved by transfection of cells in the epithelium of the enamel organ with the oncogene SV40 virus and has been named LS8.29 The second model consists of standardized primary cultures of epithelial cells of the enamel organ of human fetuses of 21 weeks of age.30 The latter model represents the ideal in vitro model for the study of fluorosis, as it comes from human cells and expresses more bookmarks than the mouse line; however, sampling has ethical implications and technical difficulties that make the LS8 cell model the more convenient and the most widely used.
As to in vivo models, some mammals have been used, including rats,31 mice,32 hamsters,33 rabbits34 and higher species, such as pigs35 and sheep.36 The rat model has proven to be the most appropriate for the study of dental fluorosis,31 since the incisors of rodents erupt continuously, and a single tooth can show the different stages of enamel development; in addition, there is evidence that the levels of F- plasma required for the appearance of fluorotic defects in enamel are very similar in humans and other animals.25,31,37,38
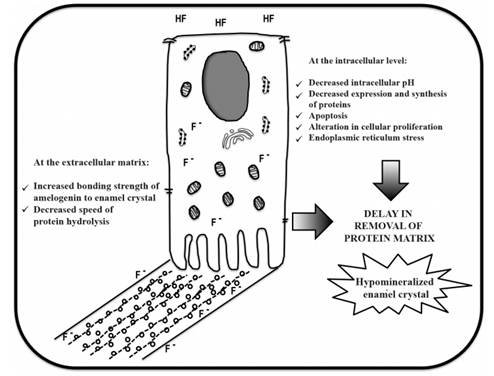
At the molecular level, dental fluorosis is a consequence of the delay in the removal of proteins from the extracellular matrix, mainly during enamel maturation.
The induction of fluorosis in animal and cell models has enabled to determine the extent to which the F- circulating in plasma in excessive concentrations during amelogenesis has deleterious effects on the different stages, including the secretion stage.39 It has been reported that at this stage fluoride induces alterations in the vesicular transport of ameloblasts40 and in the intracellular degradation of proteins of the matrix by the lysosomal system.41,42 However, experimental studies on fluorosis have focused specifically on the stage of enamel maturation (which includes the orderly sequence of crystal growth, proteolytic digestion by different enzymes, and absorption of protein residues), as it has proven to be the most sensitive to the negative effects of F-. This is based on in vivo studies performed in rats, which have demonstrated that the consumption of high amounts of F- delays the elimination of proteins (especially amelogenins).24,25 A reduced capacity of amelogenin elimination triggered by F- prevents the thickening of enamel crystals and leads to incomplete mineralization.43
In the same stage of maturation, in which the ameloblasts regulate pH by secreting bicarbonate and using ionic transporters to absorb protons from the matrix,44 the large number of protons (H+) released as a result of the high rate of precipitation of enamel crystals produce fluctuations of pH (from neutral to acid),45 and the presence of high concentrations of F- in an acidic environment has deleterious effects, as will be discussed later in this review. While the effect of F- on maturation is critical, its adverse effect on the other stages is not negligible and could be cumulative, considering that the severity of the fluorosis is linked to a sustained and prolonged exposure.43
Effects of fluoride on the physiology of ameloblasts
It has been reported that a concentration of 10 µM of F- produces a decrease in the expression of MMP-20,46,47 concentrations of 10 to 20 µM of F- produce an increase in apoptosis, and concentrations above 1 mM produce alterations in cell proliferation.47 In addition, concentrations of 120 µM of F- reduce the expression of messengers of amelogenin, ameloblastin, enamelin, and MMP-20, as well as factors of vascularization, such as the endothelial growth factor (VEGF), monocyte chemoattractant proteins (MCP-1) and the interferon inducible protein (IP-10).48
The pH regulation is fundamental for crystal growth: the precipitation of ions during enamel maturation releases a large amount of protons (H+), followed by reaction [10Ca2+ + 6 HPO42- + 2H2 O Ca10 (PO4)6 (OH)2 + 8H+], and the pH of the extracellular matrix goes from neutral to slightly acidic.45 These pH changes are reflected in the alteration of the morphology of these cells, which show rough ends in the presence of an acid pH and smooth ends in the presence of a neutral pH. During the normal development of enamel there is an alternation of these morphologies; however, in vitro studies have shown that the modulation between smooth and rough morphology become slow, with predominance of the rough one.47 therefore, as a result of the presence of fluoride, the pH of the extracellular matrix remains acid for a long time.
The exposure of LS8 cells to concentrations of 250-2000 µM of F- has provided interesting observations: the increase in the concentration of protons (H+), in the presence of a high concentration of ions F-, leads to the formation of hydrofluoric acid (HF), which is absorbed by the cells and causes severe changes in cellular metabolism.49 These findings suggest an interesting hypothesis: an excess in cytoplasmic F- in ameloblast induces stress in the endoplasmic reticulum and activates a defense called “unfolded protein response” (UPR), which decreases the synthesis and secretion of KLK-4, essential for the elimination of the protein matrix of amelogenin and the final maturation of enamel prisms.50,51,52
When F- passes through the cytoplasmic membrane toward the mineralization front, another chain of effects, demonstrated by recent in vitro studies describing the massive arrival of F- in the mineralization front, produces a hypermineralized layer of enamel which could act as a physical barrier that would prevent the diffusion of ions and proteins to the subsurface of the mineralization front.53 This molecular event could hinder the entry of “raw material” necessary for full mineralization of crystals and thus contribute to the hypominealization of fluorotic enamel.
Effects of fluoride on the activity of proteases of the extracellular matrix
It is widely known that F- inhibits the activity of proteases of the extracellular matrix of enamel. Since the number of studies is limited, the results have failed to demonstrate a direct inhibition of the enzyme activity,54,55,56 and therefore F- has been discarded as an inhibitor of proteases in the pathogenesis of fluorosis.
Effects of fluoride on the kinetics of biomineralization
The incorporation of F- in enamel crystals during their formation increases the binding strength of amelogenin to the crystal57 and reduces its hydrolysis.58 This evidence has been gathered in trials with recombinant amelogenin bound to synthetic hydroxyapatite with F- concentrations like those found in human teeth with fluorosis. The binding of proteins to crystals with high content of F- can possibly trigger changes in their conformation, thus “hiding” some cleavage sites and decreasing access to the proteases,57 reducing the speed of removal of matrix proteins and preventing the thickening and maturation of the crystal. These studies provide sufficient evidence to suggest that dental fluorosis is a consequence of the delay in the removal of proteins during the stage of maturation of enamel. In addition, proteins may possibly be retained in erupted enamel.
Based on this logic, some studies have been conducted on the retention of amelogenin in erupted enamel with fluorosis.58,59 The low protein content of erupted enamel (< 1% in healthy enamel) and the difficulties to extract proteins trapped in the mineral matrix have limited the studies aimed at extracting, identifying, and quantifying the protein material of enamel.
In order to expand the study of dental fluorosis in Colombia, at the Unidad de Investigación en Caries (UNICA) we standardized a method to extract and identify enamel proteins through liquid chromatography along with mass spectrometry. The method was applied to a sample of teeth from Colombian patients.60 Using this method, we compared the proteins identified in erupted enamel of healthy teeth and teeth with fluorosis. Our analysis showed amelogenin, ameloblastin, and enamelin-the latter more frequently in fluorotic enamel, suggesting a possible role of this protein in the events that trigger fluorosis-. In addition, through the relative quantification of identified peptides of amelogenin, we found no differences in protein content between healthy enamel and enamel with fluorosis.11 The available reports on this subject are contradictory,58, 59, 61 and to date we cannot speak of retention of proteins in fluorotic enamel but of an alteration in speed for their removal, which slows down the maturation process of enamel crystal.43
Figure 1 summarizes the available evidence on the cellular and biochemical mechanisms reported to date, possibly related to the pathogenesis of dental fluorosis.
CONCLUSIONS AND EXPECTATIONS
The cellular and molecular mechanisms by which dental fluorosis occurshavenotbeen fully explained. Nor is there consensus on the biologically relevant concentrations of F- that produce dental fluorosis in humans. In vitro and in vivo models have shown that high steady concentrations of F- have harmful effects on ameloblasts. These deleterious effects are proportional to the doses of F- used and decreases the capacity of ameloblasts to synthesize and secrete proteins, especially at the maturation stage. The susceptibility of this stage in particular may be due to pH fluctuations experienced by ameloblasts due to a high concentration of protons released during the precipitation of crystals. While F- has been thought to be a direct inhibitor of MMP-20 and KLK-4 proteases (as a possible cause of protein retention), the available evidence to date allows discarding this hypothesis. For now, it is widely known that F- affects the kinetics of biomineralization, slows down the hydrolysis of proteins, and interrupts the process of elimination of the protein matrix, triggering the incomplete mineralization of enamel crystals and producing porous enamel-which is typical of dental fluorosis.
Further studies on the pathogenesis of dental fluorosis are expected to provide evidence to the analysis of biologically relevant concentrations of fluoride (e.g., fluoride in plasma of inhabitants from fluorosis-endemic areas) and thus align them to in vivo and in vitro studies. It is also important, under such concentrations, to carry out studies on other effects of F- on the cellular physiology and the kinetics of biomineralization in vitro to fully elucidate the mechanisms that lead to this defect and to replicate studies that-in the presence of contradictory evidence-confirm whether the enamel with fluorosis shows retention of proteins.











 text in
text in