INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by progressive loss of cognitive functions.1 Its prevalence is high: about 10 to 12% of the world’s population over 65 years suffer from the disorder, and this number is expected to grow exponentially.2 As a result, the demand for dental services has increased, making it necessary to have a better understanding of the oral health needs and conditions of individuals with AD.
There are two forms of manifestation of the condition: sporadic (SAD) and familial (FAD) Alzheimer’s disease. The pathological anatomy is similar in both forms and is characterized by neurofibrillary tangles, senile plaques, granulo-vascular degeneration, and gliosis. However, FAD develops at an earlier age than SAD (before the age of 65).3
Various chromosomes have been associated with early onset of familial Alzheimer’s disease: 21, 14, 19 and 1.4The Grupo de Neurociencias de Antioquia (GNA) has identified and studied the world’s largest population with this disease, associated with the E280A mutation of chromosome 14. This cluster is composed of 42 extensive genealogies with more than 5,000 heirs, distributed throughout the department of Antioquia and the country. This mutation is caused by the substitution of glutamic acid by alanine in codon 280 of gene presenilin-1 on chromosome 14, and thus is known as mutation E280A, or paisa mutation (referring to the way people from Antioquia are called). This population has gained importance in the study of dementias worldwide, since it is the largest family group affected by FAD.5
The evidence reported in the literature regarding oral and dental health in people with AD identifies significant changes in oral conditions, such as hyposalivation, susceptibility to risk of infection, burning mucosa and tongue, taste alterations, dyslalia, swallowing alterations, dry mouth syndrome, fissures, and ulcerations in mucosa and tongue.6 However, all these findings have been identified in the sporadic form of the disease. No study has been conducted in FAD patients.
This study aimed to describe the salivary characteristics of people with early familial Alzheimer’s disease, seeking to detect saliva alterations that can cause changes in the oral microbiome. This will enable the implementation of oral health care protocols in this population before the appearance of signs and symptoms of AD.
MATERIALS AND METHODS
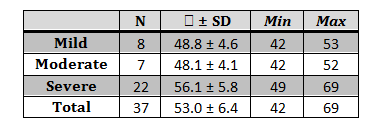
Through a descriptive transversal study, all the subjects registered in the databases of Grupo de Neurociencias de Antioquia (GNA) were contacted. All the subjects were residents in the Metropolitan Area of Valle de Aburrá. Initially, the study universe included 41 subjects (N = 41). One subject died before the sample was taken and three refused to participate in the study, leaving 37 subjects with familial Alzheimer’s disease who were evaluated, most of them from Medellín and Angostura. A preliminary interview was made, recording the subjects’ demographic data, like age, socioeconomic stratum, and years of schooling, as well as clinical aspects, such as medicine intake. Disease severity was diagnosed based on medical criteria and neuropsychological tests, such as Mini Mental State Examination (MMSE), family (FC) and patient memory complaints (PC), global deterioration scale (GDS), Functional Assessment Staging (FAST), and evocation. Table 1 shows the criteria for classification of disease stage.7
Table 1 Classification of Alzheimer’s disease stage based on some neuropsychological tests.7
| Stage | MMSE | GDS | FAST |
|---|---|---|---|
| Mild | 30-23 | 1-3 | 1-3 |
| Moderate | 23-12 | 4-5 | 4-5 |
| Severe | 12-0 | 6-7 | 6a-7f |
Participants and caregivers were contacted through the GNA. Home visits were made, explaining the purpose of the study and the freedom to participate in it. The informed consent was signed and approved by the Ethics Committee of the School of Dentistry of Universidad de Antioquia.
For saliva sampling, the caregivers were provided with the following instructions before samples were taken: avoid the consumption of solid foods one hour before the test and perform oral and dental hygiene half an hour before the evaluation. Sampling was performed between 09:00 h and 11:00 h to minimize the variations associated with the circadian cycle.
Clinical examination
Participants were asked to open the mouth to observe oral health status. The total number of teeth in the mouth and the use of dentures were recorded, establishing prosthesis type in three categories: fixed, removable, and full.
Salivary evaluation
Each participant was asked to chew a piece of sterile wax and tilt the head down, using the manual subtraction method with sterile plastic Pasteur pipettes to collect saliva from the floor of the mouth and bringing the sample to 50 mL Falcon® tubes (VWR International, Pennsylvania) for transportation. The amount of saliva was recorded (mL) for 5 minutes, calculating saliva secretion rate. Samples were collected until obtaining at least 5 mL of saliva, and all the other tests were conducted in the Microbiology and Oral Histopathology Laboratory of Universidad de Antioquia School of Dentistry. The samples were transported in a portable icebox for analysis. The Ericsson method was used to evaluate salivary buffer capacity.8 To this end, the initial salivary pH of 1 mL of saliva was measured using a WTW 330 pH-meter (Weight Watchers International, Inc. New York). Immediately afterwards, each sample was added 3 mL of 0.005 N hydrochloric acid and mixed in magnetic stirrer, evaluating final pH 20 minutes later.
Microbial count
Microbial count was performed using the total saliva sample obtained from participants. Serial dilutions were made in glass vials (Thermo Ficher Science, Massachusetts, US) using the total saliva sample in Brain Heart Infusion (BHI) (Oxoid, Basingstoke, UK) with 0.9 mL of BHI, adding 0.1 mL of total saliva in 0.9 mL BHI for 10-1 dilution. Dilutions of 10-2 to 10-5 were prepared, adding 0.1 mL of the preceding dilution; the vials were stirred in vortex in between dilutions.
Streptococcus mutans was isolated taking 0.1 mL of dilutions 10-4 and 10-5, using a sterile swab to spread in Petri boxes (Waldner Laboreinrichtungen GmbH & Co, Allgäu) with mitis salivarius agar (Beckton & Dickinson, NJ, US) prepared with 20% sucrose, 1% tellurite and 0.1% bacitracin. The boxes were incubated in a microaerophilic environment at 37 °C for 72 hours, interrupting the incubation at this time to count colony forming units. The culture for the search of Candida albicans was prepared in sabouraud dextrose agar (Merck, Darmstadt), taking 0.1 mL of the total saliva sample with the surface planting technique. It was incubated in microaerophilic environment at 37 ºC for 72 hours, recording the number of colonies afterwards.
Lactobacillus Spp was isolated using 25 mL of rogosa agar (Merck, Darmstadt) with the deep planting technique to simulate an anaerobic environment. To that end, 1 mL of dilution 10-3 was added, incubating for 72 hours in an anaerobic environment at 37 ºC. The results were interpreted and recorded.
The number of colonies of each species was counted using a stereomicroscope MS-2 20x (Optika, Ponteranica BG, Italy) and a colony counter (Acequilabs, Bogotá, Colombia). These microorganisms were selected as they are an important part of the pathogenic flora associated with the most prevalent diseases in the mouth.
The results were analyzed 72 hours later using the Frost diagram. The number of bacteria was recorded according to the colony forming units by mL (CFU/mL); it was considered as a “negative culture” if no bacterial growth was present, and “uncountable” if the number of colonies in agar was too large to perform an accurate count.
Statistical analysis
Data were analyzed with version 23.0 of software IBM-SPSS Statistics for Windows (Armonk, NY: IBM Corp.), conducting descriptive analysis with summary measures (average, standard deviation, minimum and maximum) for all quantitative variables. Qualitative variables were described with absolute and relative frequencies expressed in percentages. An exploratory statistical analysis was conducted using the Shapiro-Wilk test to evaluate the normality of the salivary variables. The ANOVA test was used to compare the age and salivary variables of AD patients against disease stage. Student’s t-test was used to compare the use of medications against salivary variables. In addition, the Pearson correlation coefficient was used to evaluate the relationship among salivary variables. A significance level of 0.05 was always used for all exploratory statistical analysis.
RESULTS
Demographic profile
We assessed 37 participants, 15 males (40.5%) and 22 females (59.5%), diagnosed with familial Alzheimer’s disease due to E280A mutation in different stages: mild: 8 (21.6%), moderate: 7 (18.9%) and severe: 22 (59.5%). The average age of participants was 53 ± 6 years, with 54 ± 5 years in average for female and 51 ± 5 for male. Age distribution changes with respect to disease stage; average age was higher in the severe stage, with statistically significant differences with respect to the moderate and mild stages-p values < 0.04 and 0.05 respectively (Table 2).
Most participants (94.6%) were from socioeconomic strata 1, 2 and 3, and the others belonged to stratum 4. The participants’ average schooling was 8.0 ± 4.7 years.
The clinical evaluation showed that participants had an average of 15.3 ± 10.8 teeth. The sample was heterogeneous in terms of this variable, since there were edentulous participants (18.9%) to participants with complete permanent dentition (5.4%). 24.3% of subjects used some type of prosthesis, mostly upper or lower removable partial prostheses (10.8%). In the mild, moderate and severe stages, participants had 23 ± 9.6, 18 ± 10.6 and 12 ± 9.7 teeth, respectively, with a Pearson correlation coefficient of 0.45.
Full saliva test
1. Stimulated saliva secretion rate (SSSR)
The average stimulated saliva secretion rate was 0.59 ± 0.41 mL/min. 45.9% of the sample had decreased SSSR.9 With an average of 54.4 ± 7.7 years and consumption of 1.9 ± 1.2 medicines, these were in moderate to severe disease stage, while those with adequate secretion rate were 51.9 ± 6.9 years in average, took 2.3 ± 1.3 medicines, and were in mild to moderate disease stage. Table 3 shows the saliva secretion values according to disease stage, with statistically significant differences in salivary secretion rate between the mild and moderate stages and the mild and severe stages, with p values of 0.003 and 0.000 respectively.
2. Salivary buffering capacity (SBC)
An initial average pH of 7.0 ± 0.7 was found. SBC was decreased in 83.9% of participants, with an average pH of 3.5 ± 0.9 after subjected to hydrochloric acid, indicating a value below the critical salivary pH of 5.
Figure 1 presents a dot scatter plot showing the low correlation of the variables: salivary buffering capacity and salivary secretion rate with a Pearson correlation coefficient of 0.277, suggesting that the secretion rate is independent of the buffering capacity.
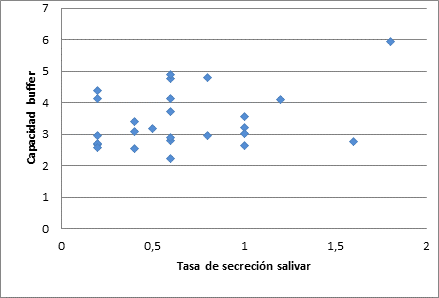
Figure 1 Correlation between buffering capacity and salivary secretion rate in Alzheimer’s disease patients. [Traducción de la figura: Vertical: Buffering capacity. Horizontal: Saliva secretion rate]
3. Microbial count
The evaluation of microbial counts in saliva showed that the average CFU’s of S. mutans was 3.39*106 CFU/mL, with values ranging from 0 to 2.4*107 CFU/mL. The Lactobacillus Spp count yielded an average of 2.58*104 CFU/mL, with values ranging from 0 to 1.5*105 CFU/mL. On the other hand, the C. Albicans culture showed a high average count (> 102 CFU/mL) in 73% of participants.
Table 3 Variables of the salivary analysis according to disease stage.
| n | X ± SD | ||||
|---|---|---|---|---|---|
| Saliva secretion rate mL/min | Mild | 8 | 1.14 ± 0.38 | ||
| Moderate | 7 | 0.59 ± 0.21 | |||
| Severe | 22 | 0.40 ± 0.28 | |||
| Total | 37 | 0.59 ± 0.41 | |||
| Buffering capacity | Mild | 8 | 3.93 ± 1.21 | ||
| Moderate | 7 | 3.47 ± 0.53 | |||
| Severe | 16 | 3.20 ± 0.77 | |||
| Total | 31 | 3.45 ± 0.89 | |||
| S. Mutans count (CFU/mL) | Mild | 8 | 3.19*106 ± 5.89*106 | ||
| Moderate | 7 | 5.94*106 ± 9.23*106 | |||
| Severe | 22 | 2.65*106 ± 4.68*106 | |||
| Total | 37 | 3.39*106 ± 5.94*106 | |||
| Lactobacillus count (CFU/mL) | Mild | 8 | 4.43*104 ± 3.96*104 | ||
| Moderate | 7 | 2.51*104 ± 5.52*104 | |||
| Severe | 22 | 1.93*104 ± 2.35*104 | |||
| Total | 37 | 2.58*104 ± 3.52*104 | |||
Types of medications and association with salivary variables
Table 4 shows the types of medication used by the participants and percentages of use. Most medications were anticonvulsants and antipsychotics, which are largely associated with decreased salivary secretion.
Table 4 Summary of medications used by AD patients and percentages of use
| Cholinesterase inhibitors | Anticonvulsants | Antipsychotics | Antidepressants | NMDA receptor antagonists | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | ||||||
| Rivastigmine | 16.2 | Valproic acid | 48.6 | Quetiapine | 43.2 | Trazodone | 21.6 | Memantine | 13.5 | |
| Donepezil | 10.8 | Clonazepam | 5.4 | Olanzapine | 16.2 | Fluoxetine | 8.1 | |||
| Gabapentin | 2.7 | Clozapine | 2.7 | Sertraline | 8.1 | |||||
| Carbamazepine | 2.7 | Haloperidol | 2.7 | Escitalopram | 2.7 | |||||
| Levetiracetam | 2.7 | Levomepromazine | 2.7 | |||||||
| Phenytoin | 2.7 | |||||||||
| Phenobarbital | 2.7 | |||||||||
The salivary variables evaluated in this study showed a statistically significant difference (p < 0.005) between the use of antidepressants and salivary secretion rate, with no influence on the other salivary variables under analysis. On the other hand, a statistically significant difference was found between the use of anticonvulsants, salivary secretion rate (P < 0.003), and saliva buffering capacity (p < 0.009) (Table 5).
Table 5 shows the behavior of the salivary variables with the different groups of medicines taken by participants.
Table 5 Medications and association with salivary variables
| Salivary secretion rate | Buffering capacity | S. Mutans count | Lactobacillus count | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Medications | Use | n | X ± SDp | 𝑿 ± SDp | 𝑿 ± SDp | 𝑿 ± SDp | |||||
| Cholinesterase inhibitors | No | 27 | 0.53 ± 0.40 | .005 | 3.40 ± 0.88 | .79 | 3.06*106 ± 5.87*106 | 1.11 | 2.11*104 ± 2.87*104 | 3.01 | |
| Yes | 10 | 0.75 ± 0.43 | 3.57 ± 0.96 | 4.26*106 ± 6.39*106 | 3.84*104 ± 4.82*104 | ||||||
| Anticonvulsants | No | 16 | 0.69 ± 0.40 | .003 | 3.62 ± 0.88 | .009 | 3.69*106 ± 6.89*106 | . 193 | 3.23*104 ± 3.51*104 | .905 | |
| Yes | 21 | 0.51 ± 0.41 | 3.31 ± 0.90 | 3.16*106 ± 5.28*106 | 2.09*104 ± 3.53*104 | ||||||
| Antipsychotics | No | 16 | 0.59 ± 0.37 | .028 | 3.56 ± 0.84 | .071 | 2.67*106 ± 4.58*106 | 1.26 | 2.57*104 ± 3.42*104 | .125 | |
| Yes | 21 | 0.60 ± 0.45 | 3.36 ± 0.95 | 3.93*106 ± 6.87*106 | 2.59*104 ± 3.67*104 | ||||||
| Antidepressants | No | 23 | 0.51 ± 0.32 | .099 | 3.47 ± 0.80 | .510 | 3.74*106 ± 6.61*106 | . 340 | 3.09*104 ± 3.94*104 | .132 | |
| Yes | 14 | 0.73 ± 0.52 | 3.42 ± 1.06 | 2.81*106 ± 4.82*106 | 1.74*104 ± 2.61*104 | ||||||
| NMDA receptor antagonists | No | 32 | 0.61 ± 0.43 | .297 | 3.44 ± 0.89 | .220 | 3.45*106 ± 6.26*106 | . 505 | 2.65*104 ± 3.62*104 | .174 | |
| Yes | 5 | 0.48 ± 0.31 | 3.49 ± 1.12 | 2,96*106 ± 3,70*106 | 2,12*104 ± 3,04*104 | ||||||
DISCUSSION
This study assessed 37 participants with the E280A mutation for early familial Alzheimer’s disease. This and other genetic forms constitute about 1% of the disease worldwide.10 The sample studied is therefore representative, considering the low prevalence of this form of the disorder. Epidemiological AD studies report greater prevalence levels of this disease in women than in men.2 The results of the present study show a similar behavior among the individuals studied, since 59.5% of the sample were women aged 2.6 years older than men in average.
The study’s demographic analysis showed low socioeconomic and schooling levels.11
The literature has suggested that there is an increased risk of Alzheimer’s disease in the presence of lower schooling and socioeconomic levels.12 However, the sociodemographic variables play a secondary role in the genetic form of the disease.13,14
Other studies analyzing oral health problems in people with AD fail to specify the type of Alzheimer’s disease under study, and participants in those studies are usually over 60 years of age,15,16 a time when the oral-dental health conditions may be more altered due to greater organic deterioration. This study is then valid as it indicates the type of Alzheimer’s disease and assesses a sample of younger subjects.
The clinical examination evaluated the participants’ number of natural teeth, finding out a prevalence of edentulism of 18.9%, which is a low value compared with other studies, ranging from 50 to 70%.17,18 The present study suggests that there is a positive correlation between number of teeth and disease stage, with a lower proportion of teeth in subjects with moderate to severe familial AD. In a group of 60 patients (30 with AD and 30 in the control group) evaluated at Universidade Estadual Paulista Júlio de Mesquita Filho (Brazil), Ribeiro et al (2012) found higher DMFT values among people in moderate and severe disease stages (p = 0,0191).15 However, in a study conducted in the same country, Machado et al found no statistical significance between disease severity and number of natural teeth (p = 0.346).16
The saliva test showed that the average stimulated saliva secretion rate was 0.59 ± 0.41 mL/min. These findings are slightly lower than those by Leal et al in a study conducted in 40 volunteers (20 of them with senile dementia), in which the stimulated saliva secretion rate was 0.69 ± 0,39.6 It has been reported that values from 0.5 to 0.7 mL/min of stimulated saliva flow are indicators of sialopenia or hyposalivation.9,19 The results of the present study show that 45.9% of the sample has decreased salivary secretion rate. This decrease has been explained in some studies,20,21,22 in which medicine intake in people with AD has been associated with reduced saliva flow. However, the present study showed that patients with reduced salivary secretion (in moderate to severe stages) were older and took less medicines than those with adequate salivary secretion (in a mild to moderate stage). Thus, in comparing participants with unaltered saliva rate with participants with reduced salivary flow, it may be suggested that other variables besides the pharmacological factors analyzed in this study, such as age and disease severity, can influence the behavior of the physiology of saliva.
Furthermore, Lopez-Jornet and Bermejo-Fenoll explain the influence of age on salivary flow, indicating that the parenchyma of the salivary glands undergoes degenerative changes with age, which may explain the low salivary flow in older people.23 This assertion reinforces the findings of the present study, in which older participants showed reduced saliva flow. On the other hand, a study by the University of Oxford shows that non-medicated AD patients present hyposalivation, limited to the submandibular gland, of unconfirmed cause.24 Therefore, the causes of such hyposalivation in AD patients might need new scientific corroboration.
In healthy conditions, it has been reported that the pH of saliva in rest remains at a narrow range, between 6.7 and 7.4.25 In evaluating the initial pH of participants, values of 7.021 ± 0.743 were found, suggesting an adequate salivary pH. The buffering capacity of saliva was reduced in most participants, as an average pH of 3.449 ± 0.89 was found after submitting it to hydrochloric acid, a value that expresses a reduced SBC, unable to stabilize the critical pH of the mouth, which is 5.5.26 There is a positive association between salivary flow reduction and decreased buffering capacity.27 However, participants with no saliva flow alteration show a significant decrease in saliva buffering capacity, as shown in Figure 1-suggesting that the familial AD patients evaluated in this study have a decreased SBC, regardless of the saliva secretion rate.
Within the oral microbiota, S. mutans plays an important role in the onset of dental caries, being the most frequently isolated microorganism in human carious lesions.28,29 Counts exceeding 105 CFU/mL of S. mutans indicate increased risk of tooth decay.30 Lactobacillus Spp is another bacterium involved in the cariogenic process; normally, a low count of this microorganism is found in saliva but it increases when S. mutans is established in the oral cavity.31 Hidalgo et al have reported that the high level of lactobacilli infection (> 106 CFU/mL) is associated with high caries activity.32
The present study shows that the average CFU of S. mutans was 3.3878*106 CFU/mL, with values ranging from 0 to 2.4*107 CFU/mL, indicating a high count of this microorganism in FAD patients. Edentulous participants (18.9%) showed low or negative growth of this bacterial species, which usually colonizes solid surfaces of the oral environment.33 On the other hand, the average Lactobacillus Spp count was 2.5784*104 CFU/mL, with values ranging from 0 to 1.5*105 CFU/mL, in agreement with reference values.
The presence of dental bacterial plaque has been associated with high counts of S. mutans and Lactobacillus.34 The decreased cognitive and motor capacity in AD patients hinders oral care practices and limits the effective removal of dental plaque deposits, a condition that undeniably predisposes to the development of dental caries in these people. However, the single colonization of these microorganisms, associated with cariogenic processes, is not a predictor of active lesions on its own, and therefore an individualized analysis should be carried out to confirm the presence of active lesions clinically.
Another species found in the microbiological analysis was C. albicans, a frequent colonizer of the oral cavity; values higher than 102 CFU/mL indicate a high count in the oral cavity.35 This study found a high count of this species in 73% of participants; the use of fixed and removable prostheses was associated with high levels of growth of the fungus. This finding is comparable to that by Müjgan Güngör et al in a study conducted in Turkey by clinical inspection, detecting a high incidence of denture-associated stomatitis.36 However, the present study shows that 56.7% of participants with no prosthesis present an increased count of C. albicans. Therefore, the use of these devices cannot be associated exclusively with the presence of C. albicans infections in FAD patients.
On the other hand, authors like Taybos report that low salivary flow velocity can explain the increased number of S. mutans and Lactobacillus, favoring the development of tooth decay. This can be explained because saliva is involved in dental integrity through its actions of mechanical cleaning, carbohydrate clearance, ionic medium regulation, and supply of remineralization capacity, being essential for the acid-base balance of dental plaque.37 However, according to Dowd, there is little evidence that variations in salivary flow can influence the development of new caries lesions.38
When considering the type of medications and its association with salivary variables, it should be remembered that the first line of pharmacological management in AD patients is the use of cholinesterase inhibitors (CI’s), because during the pathophysiology of this disease there is a reduction of brain levels of acetylcholine and loss of cholinergic neurons. These medications have shown to be effective in preserving cognitive functions in patients with mild to moderate AD. CI’s have been associated with increased saliva production. However, when cognitive impairment has reached higher levels, the therapy with cholinesterase inhibitors does not offer additional benefits, so patients in more advanced stages of the disease do not often use CI’s. The adjuvant therapy in AD seeks to minimize behavioral symptoms and mood disorders by using psychotropic medications such as antipsychotics, antidepressants, anxiolytics, and anticonvulsants. The most common oral side effect of this type of medication is xerostomia;20 this could explain why people in more advanced stages of the disease show lower salivary deposits (see Table 3). On the other hand, this study found a significant relationship between low salivary secretion rate and the use of antidepressants and anticonvulsants-which in some studies and systematic reviews have been associated with salivary reduction.6,20-22
The main limitation of this study is the lack of a control group, which could add analytical elements in the study variables. On the other hand, the sample size limits the statistical analysis to some extent. Similarly, the limited amount of scientific literature addressing the subject makes it difficult to establish parameters of comparison.
CONCLUSIONS
People with early familial AD who participated in this study showed altered salivary characteristics, such as salivary secretion rate, saliva buffering capacity, and an increase in oral bacterial flora. This reduction or alteration of salivary characteristics has been widely attributed to the consumption of medicines. However, this study suggests that other factors could explain the hyposalivation in FAD patients, like age and disease severity, which cause higher levels of dependency and therefore loss of self-care capabilities.
The literature review evidences the lack of oral health studies in this population, which constitutes the largest family group affected with FAD worldwide, so this article can be considered an innovation.
The present study recommends conducting research in non-diseased and unmedicated populations with the autosomal dominant gene for early familial AD, in order to detect early changes in the variables studied.











 text in
text in