INTRODUCTION
Due to the dynamic conditions of the oral environment, enamel and exposed dentin are subjected to constant fluctuations of temperature, moisture, presence of biofilm and pH changes.1 The latter two are stimulated by the intake of carbohydrate- rich foods, which promotes the adhesion, proliferation and activity of various cariogenic microorganisms which release acids that reduce dental hydroxyapatite into more soluble apatite. The cumulative effect of the aforementioned alterations and the influence of other factors such as time, reduction of the buffering capacity of saliva, and deficient oral hygiene favor the onset of incipient dental caries lesions.2,3 However, the oral environment is dynamic and the re-incorporation of lost minerals occurs alternately to the dental demineralization processes with the help of saliva and its contribution of Ca2+ and PO43- ions through a complex process called dental remineralization.4
Currently, there are several adjuvant methods to speed up this process, prevent and control the progression of incipient carious lesions, also called white spot lesions (WSLs). Sodium fluoride (NaF) is the most commonly used agent and can be supplied systemically through fluoridated water or in common diet foods. It can also be applied topically through toothpastes, mouthwashes or gels/varnishes during dental consultation.5,6 The bioavailability of NaF in saliva causes its precipitation and stability on dental structure, delaying the demineralization process and promoting dental remineralization process.7 However, there have been discussions related to its use, due to the low solubility in the presence of Ca2+ and PO4 3- ions in saturated solutions.1,7 In addition, the rapid deposition of NaF in the surface layer of the carious lesion limits a deep remineralization.8 The toxic effects of NaF at high concentrations have been controversial, due to the high risk when taking low doses from multiple sources. In fact, the cumulative effect of NaF during the early stages of life is sufficient to cause dental fluorosis.9,10
Another preventive/therapeutic alternative tested in in vitro, in situ and in vivo studies and used in recent years is the casein phosphopeptide-amorphous calcium phosphate nanocomplex (CPP-ACP).11,12 This nanocomplex is an agent that saturates saliva and biofilm, favoring the dental remineralization process by providing Ca2+ and PO43- ions, which then become available, reducing the risk of hard dental tissues demineralization. Modifications to the original nanocomplex have recently been made, incorporating NaF concentrations to enhance its preventive effect. Several studies have compared the remineralizing action of this complex and NaF, traditionally used in the prevention and treatment of initial caries lesions.13,14
In addition, manufacturers of CPP-ACP- based products indicate that it produces a desensitizing effect. However, its clinical superiority over other strategies has not been confirmed. Some dental desensitizers are potassium nitrate (KNO3), fluorides, glutaraldehyde, hydroxyethyl methacrylate (HEMA), arginine-calcium carbonate complex, sodium calcium phosphosilicate (Bioglass 45S5), strontium acetate, oxalate, and laser.15 Different concentrations of CPP- ACP have been incorporated experimentally into dental materials and used in adhesive laboratory procedures to promote additional benefits to dental structure.16,17 For these reasons, it is important to know the remineralizing/desensitizing benefits of CPP-ACP compared to traditional strategies, as well as the products that incorporate this technology and their clinical effectiveness based on evidence. The objective of this topic review was to present the state of the art on the casein phosphopeptide-amorphous calcium phosphate nanocomplex.
CASEIN PHOSPHOPEPTIDE- AMORPHOUS CALCIUM PHOSPHATE NANOCOMPLEX (CPP-ACP)
CPP-ACP is a nanocomplex patented by Reynolds at the University of Melbourne, Australia.18 It is made up of the phosphopeptide casein protein (CPP), which is derived from milk and it is structured by residues of phosphorylated serine and glutamic amino acid.19 In addition, it contains a precursor of dental hydroxyapatite: amorphous calcium phosphate (ACP), whose ions are phosphorylated by the serine residues in CPP.20 Several studies focus on its remineralizing and dental caries preventive effect.12-14,16,19 In ad- dition, it has been noted that it produces a desensitizing action,15 as well as additional bacteriostatic effects.20
The remineralizing effect of CPP-ACP
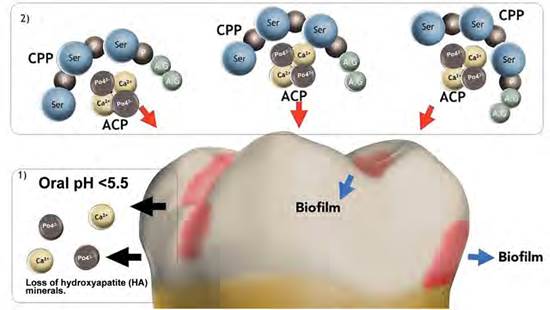
The remineralizing effect is caused by the stabilization of Ca2+ and PO43- ions that are in solution, by binding ACP to multiple amine residues of phosphorylated serine present in CPP. Thus, it enables the formation of nano-sized clusters, also called CPP-ACP nanoclusters, which increases the total surface area, as well as its interaction with biofilm and dental structure. The clusters prevent nucleation and spontaneous precipitation of Ca2+ ions, and behave like salivary proteins such as estaterin and other proline-rich proteins.21 The continued use of dental products containing CPP- ACP produces ion saturation in saliva and biofilm, making it available for subsequent precipitation in the form of ACP, favoring the dental remineralization process (Figure 1).22-24 Several laboratory studies have suggested that the remineralization process promoted by this nanocomplex decreases surface roughness and improves the micromorphological characteristics of tooth enamel following microabrasion, orthodontic stripping, bracket removal, or erosive processes.25-27
Other in vitro studies have shown that the remineralizing effect of products containing CPP-ACP nanocomplex is similar to that produced by fluorides.28,29 However, this superiority has been questioned, as recently shown through the use of laser confocal microscopy to evaluate the remineralizing potential of a fluoride varnish (Fluor Protector; Ivoclar Vivadent), a CPP-ACP- based paste (GC Tooth Mousse, Recaldent; GC Corp; Japan) and another paste based on fTCP (functionalized Tri-Calcium Phosphate) (Clinpro Toothcreme, 3M). The results showed that the fluoride varnish was more effective in decreasing the depth of incipient dental caries lesions, followed by the CPP-ACP-based paste; however, this latter was better than the one containing fTCP.30 In 2015, Vyavhare et al compared the remineralizing effect of nano-hydroxypatite (10%), NaF (1,000 ppm), CPP-ACP (10%) and deionized water (negative control) by means of Vickers microhardness and scanning electron microscopy (SEM), demonstrating that the use of nano-hydroxypatite and NaF significantly increases microhardness and promotes the reduction of superficial defects and porosities on demineralized tooth enamel.31
Source: By the authors
Figure 1 Graphic representing the process of dental demineralization/remineralization. 1) Oral pH < 5.5 induces loss of hydroxyapatite (HA) minerals. 2) Saturation of the oral environment and biofilm with Ca2+ and PO43- ions, promoting the dental remineralization process.
For these reasons, the remineralizing property of NaF has recently been integra- ted into dental products containing phosphopeptide to form a new complex called CPP-ACPF (casein phosphopeptideamorphous calcium phosphate fluoride). It should be emphasized that there are no negative interactions between the two components; on the contrary, there is a synergistic effect, providing essential components (PO43-, Ca2+, NaF) to dental structure, which is ideal to promote an integral remineralization process.32) Evaluations conducted in human enamel by means of Vickers microhardness, SEM and laser fluorescence have shown that remineralizing CPP-ACP-based pastes that incorporate 0.2% NaF (e.g., MI Paste Plus™ / GC Tooth Mousse-Plus®) increase surface microhardness, decrease the surface irregularities produced by demineralization and form more marked mineral deposits than those generated by pastes that only contain CPP-ACP.33,34 However, the remineralization of the dental surface is observed only 7 days after starting treatment with Tooth Mousse-Plus®.35 Currently, there are several dental products that contain CPP-ACP, CPP-ACPF and ACP in different presentations, such as topical pastes, gels, varnishes, xylitol- or sorbitol-based chewing gums, among others (Table 1).36-38
Table 1 Dental products containing CPP-ACP, CPP-ACPF and ACP
| Active ingredients | Trademark (Manufacturer) | Commercial presentation |
|---|---|---|
| CPP-ACP | MI Paste™ (GC) / Tooth Mousse™ (GC) | Topical paste |
| CPP-ACP | Trident Xtra Care® (Adams) | Chewing gum |
| CPP-ACP | Trident Total® (Adams) | Chewing gum |
| CPP-ACP | FUJI VII™ EP (GC) | Glass ionomer cement |
| CPP-ACPF | MI Paste plus™ (GC) / Tooth Mousse plus™ (GC) | Topical paste |
| CPP-ACPF | MI Varnish™ (GC) | Varnish |
| ACP | NiteWhite™ (Phillips) | Tooth bleaching gel |
| ACP | DayWhite™ (Phillips) | Tooth bleaching gel |
| ACP | Aegis® Ortho (Bosworth Co) | Dental adhesive |
| ACP | Aegis® Pit and Fissure Sealant (Bosworth Co) | Pit and fissure sealant |
| ACP | Relief® ACP (Phillips) | Tooth desensitizing gel |
| ACP | Enamelon® (Premier) | Toothpaste |
Dental desensitizing mechanism of CPP-ACP
The precipitated Ca2+ and PO43- ions from ACP are spread through the phosphorylated fibrils of the exposed intertubular dentin collagen, promoting the formation of apatite.39 However, both ions are mainly integrated into hypermineralized peritubular dentin, creating deposits at the intratubular level (Figure 2). This strategy seeks to partially block the external stimuli that influence the hydrodynamic behavior of dentin-pulpar fluid, consequently it decreases dentin hypersensitivity.40 However, the blocking action in the dentin tubules is partial, similar to that produced by other desensitizing strategies such as the arginine-calcium carbonate complex or strontium acetate.41
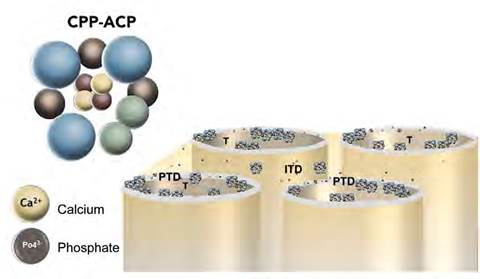
Figure 2 Image representing the blocking of exposed dentinal tubules and mineral precipitation from CPP-ACP. T: exposed dentin tubule; PTD: peritubular dentin; ITD: intertubular dentin
The stability of CPP-ACP mineral deposits formed on exposed dentin could be affected by the action of brushing, dietary acids, and phosphatases produced by oral bacteria, causing loss of phosphate groups in the phosphoserines of nanocomplex peptides.42,43 Therefore, multiple applications of topical dental products incorporating CPP- ACP are needed to promote the formation of significant precipitates of Ca2+ and PO43-
Bacteriostatic mechanism of CPP-ACP nanocomplex
The bacteriostatic mechanism is explained because the nanocomplex is able to bind to the biofilm matrix, saturating it with Ca2+ and PO43- ions from ACP.44 It has been observed that pastes containing CPP-ACP (10%) and glass ionomer cement (GIC) with concentrations of this complex (3%) are able to increase local pH, promoting a neutralizing effect and, masking the receptors of salivary molecules that allow bacterial adhesion. As a result, CPP-ACP slows down growth and decreases the number of colony-forming units of bacteria with high cariogenic potential, such as Streptococcus mutans.45-47 This positive effect was also observed in a short-term clinical study conducted in patients with high risk of dental caries and chronic gingivitis, concluding that the use of a paste based on CPP-ACP reduced the count of bacteria from supragingival plaque and salivary samples, even one month after discontinuing its use.48
INDICATIONS AND EVIDENCE- BASED CLINICAL EFFECTIVENESS OF CPP-ACP
Dental Caries
The first visible clinical sign of dental caries is the presence of white spot lesions (WSLs), compatible with demineralization.49 Several strategies, such as topical fluorides, resin infiltration, and CPP-ACP have been used to achieve regression and improve the aesthetic appearance of these lesions.50
In relation to the latter strategy, Memarpour et al (2015) compared the effectiveness of a fluoride varnish (22,600 ppm) (DuraShield; Sultan Healthcare, Hackensack, N.J., USA) with a paste containing CPP-ACP (GC Tooth mousse, Gc, Tokyo, Japan) on WSL regression in 140 children. Measurements of the lesion area and dmft index showed that CPP-ACP paste had a preventive effect similar to the fluoride varnish. However, prolonged use of CPP-ACP decreased the presence of such non-cavitated lesions.51 Another clinical study conducted by Andersson et al (2007) showed that WSL regression can be achieved using the same CPP-ACP paste or toothpastes containing NaF. Nonetheless, the visual evaluations showed that patients who used CPP-ACP-based paste had better aesthetic results compared to NaF group, possibly related to the presence of other minerals such as calcium and phosphate from ACP which are incorporated to dental structure.52
The risk of developing WSLs is also high in patients with fixed orthodontic appliances, because the use of wires, elastics, bands, and brackets hinders correct oral hygiene, favoring biofilm retention.53,54 In 2011, Bröchner et al conducted a controlled clinical trial in 60 adolescents who had at least one WSL following removal of orthodontic appliances. The results from quantitative ligth-induced fluorescence showed a significant size reduction on incipient dental caries lesions after 4 weeks of using CPP-ACP-containing pastes, improving the clinical aspect of the lesions.55 A systematic review included 12 controlled clinical studies that evaluated alternatives such as NaF (in vehicles like toothpaste, varnish, and mouthwash) and paste containing CPP-ACP. Overall, the latter proved to be significantly effective in preventing and regressing WSLs.56
Another group of patients at high risk of incipient dental caries lesions are those who have degenerative salivary glands diseases, underwent cancer treatment such as chemotherapy, head and neck radiation therapy, or have physical/mental disabilities.57,58 In this context, an in situ study assessed the effectiveness of three remineralizing agents (CPP-ACP, CPP-ACPF and NaF at 0.05%) in Sjogren’s syndrome patients. Several relevant aspects related to saliva were evaluated (flow rate, pH, buffering capacity and mineral concentrations), as well as biofilm pH. The study concluded that the use of CPP-ACP and CPP-ACPF caused a slight increase in saliva pH and a significant increase in biofilm pH. In addition, the analysis by energy-dispersive spectroscopy and SEM performed on samples in situ showed that the use of these alternatives increased the concentrations of Ca2+ and PO43- in the dental surface, and decreased the micromorphological defects of the enamel surface, which could be of clinical relevance to patients with this condition.59
Patients with head and neck cancer undergoing radiation therapy experience non-common dental caries, especially in the cervical, cuspid and incisal levels, characterized by rapid progression and high potential for destruction of dental tissues. Daily use of CPP-ACP and NaF in these patients tend to produce a greater preventive effect against post-radiation caries.60 However, another study showed that there was no additional positive impact of this nanocomplex in terms of dental caries prevention when associated with a 0.4% stannous fluoride-based gel in patients with nasopharyngeal carcinoma undergoing radiation therapy.61
In relation to disabled patients, Özdas et al (2015) conducted a study in cerebral palsy children aged between 3 and 8 years. First, pH measurements were performed on biofilm and saliva buffer capacity was determined. In addition, a CPP-ACP-based paste (GC Tooth mousse, GC, Tokyo, Japan) was applied, evaluating the abovementioned characteristics for a maximum period of 8 weeks. Decreased acidogenicity of biofilm and increased buffering capacity of saliva was observed since the fourth week of treatment.62 However, there were no comparisons with other remineralizing agents. Clinical evidence on these specific cases is very limited to draw conclusions.
Dental sensitivity
There are several dental desensitizing strategies such as NaF, KNO3 and strontium used in a traditional way. Also, arginine/calcium carbonate complex, bio-active glasses (Bioglass 45s5) and CPP-ACP are being used.63 In relation to the latter, several clinical studies have made comparisons with conventional methods. As an example, Mahesuti et al (2014) determined the efficacy of MI Paste™ (GC America, GC, USA) and a KNO3-based gel (UltraEZ, Ultradent Products, Inc) in decreasing dentin hypersensitivity levels. The results showed that both dental products were effective but patients treated with KNO3 experienced decreased pain within two days of treatment, while the group treated with CPP-ACP showed improvements after 7 days.64 This difference in terms of effectiveness was also reported by Madhavan et al (2012), who compared the effect of a paste containing CPP-ACPF with NaF and propolis after 1, 7, 15, 28, 60 and 90 days, concluding that although all agents reduced the levels of dentin hypersensitivity, patients treated with CPP-ACPF-based paste had to wait more days to experience a significant effect.65
It is important to note that the aforementioned cases are characterized by the presence of permeable dentin exposed to the oral environment, which enables the diffusion of KNO3 up to the nerve endings of Deep dentin, or the partial block of dentinal tubules by deposits of Ca2+ and PO43-.64-65 However, patients subjected to dental bleaching with hydrogen peroxide or carbamide-based materials generally feel transient pain during or after treatment, especially when high concentrations of bleaching agents or light sources are used.66 In these cases, dentin exposure to the oral environment is not necessarily present; therefore, pain has a different etiology and the synergistic effect of desensitizing therapies is required to improve patient experience during esthetic treatment.
Under conditions of transient post- bleaching pain, CPP-ACP-based paste had a desensitizing effect similar to 5% KNO3 and 0.7% sodium monofluorophosphate after 1 and 7 days as shown in a randomized controlled clinical study.67 Maghaireh et al (2014) also compared the desensitizing potential of this paste with 2% NaF gel in patients subjected to tooth bleaching using 35% hydrogen peroxide after 1, 3, 7 and 14 days. The study showed that CPP- ACP incorporated in the paste reduced the levels of post-treatment dental pain, but did not present additional therapeutic effects compared to other strategies.68 However, this desensitizing action in transient post-whitening pain was not observed in patients using xylitol-based chewing gums containing CPP-ACP (at lower concentration: 0.6%), compared with conventional chewing gums.69,70
There are tooth bleaching materials such as DayWhite™ and Nitewhite™ that include ACP in their chemical composition. When comparing the desensitizing effectiveness of Nitewhite and other material based on 15% carbamide peroxide, KNO3 and fluoride, there were no differences in pain levels between both treatments.71 In general, results from clinical studies suggest that CPP-ACP-based dental products (especially pastes) have a desensitizing effect; however, it does not appear to be higher than other strategies, like KNO3 or NaF.
Interaction between the CPP-ACP nanocomplex and dental bleaching materials
Besides the sensitivity experienced during and after dental bleaching, another relevant topic is related to possible interaction with agents or compounds present in dental structure, different from pigments.
In this specific case, some in vitro studies suggest that the action of CPP-ACP-based dental products does not interfere with the oxidation-reduction reactions (redox) generated between the bleaching agent and the structure of chromophores responsible for dental pigmentations; in consequence, CPP-ACP does not affect the aesthetic result of tooth bleaching.72,73 Another therapeutic alternative to in-office dental bleaching is the application of gases with hyperoxidizing potentialsuchasozone(O3),whichrepresents an allotropic form of oxygen (O2). However, by means of the spectrophotometric values of Delta L* (lightness difference), Delta A* (red-green difference) and Delta E (total color difference), it has been confirmed that the concomitant use of a CPP-ACP-based paste during dental bleaching does not affect the oxidizing potential of O3.74
The CPP-ACP nanocomplex and the mechanical properties of water-based dental cements
The remineralizing properties of glass ionomer cements (GICs) on demineralized dental regions have been widely reported in the literature.75,76 However, comparing the remineralization patterns of both options, it has been found that GICs act only on the surface of dental caries lesions, while CPP- ACP-based pastes could remineralize the entire body.16 Therefore, an in vitro study added various concentrations of CPP-ACP into a GIC, specifying that such incorporation must be controlled because concentrations higher than 8% extend the setting time, a factor that can affect the clinical use of this material.77 Nevertheless, another in vitro study showed that the addition of 1.56% (in weight) of CPP-ACP into a GIC increased the material’s tensile and compressive strength.78 Under experimental conditions, this remineralizing nanocomplex has also been incorporated into temporary eugenol zinc oxide-based cements (Freegenol™ and Temp-Bond® NE) at various concentrations. Similarly, it has been shown that there is a directly proportional relationship between the added percentage and the negative effect on some of their physical and chemical properties, such as solubility and tensile/ compressive strength.79
The CPP-ACP nanocomplex and adhesion to dental structure
The CPP-ACP nanocomplex has been used in several experimental in vitro protocols as dental surface treatment prior to the application of adhesive materials.16,17 Clinically, this seeks to seal dentin tubules, reduce post-operative sensitivity and promote the remineralization of previously demineralized dentin or enamel, through the addition of ions provided by ACP. Nonetheless, mineral deposits on dental surface could lead to negative effects on the adhesive interface, as shown by Adebayo et al (2010), who observed microscopic defects in the adhesive interface when a one-step self-etching adhesive was used on dentin previously treated with a CPP- ACP-based paste. On the contrary, these micromorphological alterations were not observed when a two-steps self-etching adhesive was applied.17
It is important to note that there is a direct relationship between the characteristics of the adhesive interface and bond strength values of these materials on dental hard tissues. Several studies have shown that prior application of pastes incorporating the remineralizing nanocomplex does not affect the bond strength of etch-and-rinse or two-steps selfetching adhesive systems on dentin affected by dental caries and sound dentin/enamel.80-84 However, when one-step self-etching adhesive systems are applied to enamel previously treated with CPP-ACP, the bond strength values of these simplified adhesive materials are significantly reduced.85 This fact can be explained because the mineral deposits of ACP on the enamel structure cannot be easily modified by the acidic functional monomers contained in one-step self-etching adhesive systems and consequently hinder the interaction and binding to this structure. Nonetheless, a different situation occurs with two steps self-etching and etch-and-rinse adhesive systems.85-87 The use of phosphoric acid promotes the formation of considerable micromorphological alterations for mineral deposits and the micromechanical interlocking of adhesive systems, without decreasing bond strength values.85,87
In general, in vitro evidence shows that the effects of using CPP-ACP prior to adhesive systems are material-dependent, with a higher tendency to keep the bond strength of etch-and-rinse adhesive systems and impair the behavior of one-step self etching adhesive systems due to the reaction of the functional monomer phosphoric radical with the cations released by the agglomerates. A similar mechanical impact has been observed when comparing conventional and self-etching resin cements, but the available evidence on the influence of CPP-ACP into these luting materials is still very limited.88
CONCLUSIONS
The use of dental products incorporating CPP-ACP has a preventive potential for dental caries, but this potential is not higher than sodium fluoride. However, there is a greater tendency to regression of enamel white spot lesions when products containing CPP-ACP or CPP-ACPF are used.
The dental desensitizing effect promoted by CPP-ACP or CPP-ACPF-based products is transient, similar or less effective than other strategies.
The experimental incorporation of CPP- ACP into dental cements should be controlled for not to affect the physical- chemical properties of the material.
The CPP-ACP pre-treatment impact on the bond strength of adhesive systems to enamel and dentin is material-dependent.














