INTRODUCTION
Treatment with implant-supported prostheses for the replacement of missing teeth has increased in recent decades, improving the quality of life for partially and totally edentulous patients.1 Implant survival rates are high but depend on several risk factors.2-8 However, an increase in the incidence of peri-implant diseases has been reported. Two peri-implant diseases have been recognized thus far: peri-implant mucositis (PIM) and peri-implantitis (PI). PIM is the early and reversible inflammation of peri-implant soft tissues, while PI is a chronic inflammatory process involving the loss of bone support around the implant.9
There are numerous risk factors that may compromise peri-implant health.10 These factors include implant-related factors (design, length and diameter, surface treatment, prosthetic connection, and corrosion),11-13 factors related to surgery and rehabilitation (surgical technique, loading protocol, type of rehabilitation, presence of occlusal overload, and excess cement),14-15 and patient-related factors (medical conditions; history of periodontal disease; microbiological, inflammatory, and genetic risk factors; cigarette smoking; emotional stress; oral hygiene habits; and compliance with supportive periodontal therapy).16-22 Treatment of peri-implant diseases represents a challenge in clinical decision-making since local and systemic risk factors may need to be individualized for each patient.
Supportive periodontal therapy in dental implant patients should focus on primary prevention of peri-implant diseases, early identification of PIM cases, and early treatment of PIM and PI to halt the progression of both conditions.23 Many systematic reviews (SRs) and randomized controlled clinical trials (RCTs) have been published regarding the treatment of peri-implant diseases. However, their findings on the efficacy of preventive treatments,21-22,24 non-surgical treatments,25-26 adjunctive therapies (anti-bacterial agents,27 photodynamics,28 air-polishing,29 and laser30) and conventional resective and regenerative surgery31-37 are controversial.
In the presence of complications in dental implant patients, decisions should be based on the available scientific evidence. Despite the existence of SRs, RCTs, and consensuses, there is still uncertainty regarding the clinical outcomes for different treatment modalities. Clinical practice guidelines (CPGs) may be useful in reaching agreements or common points in different situations (geographical and economic conditions and availability of biomaterials or technologies), especially in those areas of clinical practice where management of a disease or condition is controversial.
The Scottish Intercollegiate Guidelines Network (SIGN) regulates the development of evidence based CPGs for the National Health Service (NHS) in Scotland.38 These guidelines are mainly derived from SRs of the scientific literature, but other types of study design may be used if there is insufficient evidence. They are designed to accelerate the translation of new knowledge, reduce variations in clinical practice, and improve treatment outcomes. Although there are many consensuses on dental implants, to date no CPGs have been published using the SIGN methodology. One guideline for the management of intrabony defects has been previously published using this methodology.39
The Population, Intervention, Control, and Outcomes (PICO) question asked for this guideline development was as follows: What are the best treatment options in patients with peri-implant diseases (PIM and PI) regarding outcomes in terms of clinical parameters, satisfaction, and reduction of adverse events?
The main goal of this CPG was to guide evidence-based decision-making and recommendations for the treatment of peri-implant diseases.24
The specific objectives were:
To explore the importance of adherence to maintenance programs in implant patients and to establish the parameters that should be used to determine their frequency.
To examine the available evidence and to provide recommendations for the prevention and treatment of PIM.
To identify the available evidence and recommendations for the treatment of PI.
METHODS
Guideline developers
This guideline was developed by five authors (RM, AV, AD, AG, and VM), following SIGN guidelines and international standards.
Who benefits from this guideline?
General dentists; dental hygienists; specialists in periodontics, implantology, oral rehabilitation, and oral and maxillofacial surgery; administrative staff; and patients.
Updates
In a second phase, the implementation of this guideline will be assessed in different clinical scenarios (private clinics and/or universities), evaluating clinical outcomes, patient comfort, and the feasibility of its application. This guideline will be updated every three years by the authors and/or external individuals who may join over time.
Inclusion criteria for the guideline development
The data included were mainly based on the findings of SRs and RCTs. If any aspect of this guideline had less robust evidence for PI treatment decisions, another study design was included. The consensus of experts was considered in the recommendations. Appraisal instruments from the SIGN 50 guidelines were used for the development of this guideline, levels of evidence, and grades of recommendation. For critical reading, the Prisma, Consort, and Strobe guidelines were used for SRs, RCTs, and observational studies, respectively.
Reference results for professionals
The following variables were considered: risk factors that modify the response to treatment, importance of supportive periodontal therapy and its frequency, mechanical and antimicrobial therapy, treatment options for PIM, non-surgical or surgical treatment options, new technologies, and resective or regenerative therapy.
Exclusion criteria
Articles with low methodological quality and/or high risk of bias were excluded after applying the instruments for critical reading.
Scientific research: identification
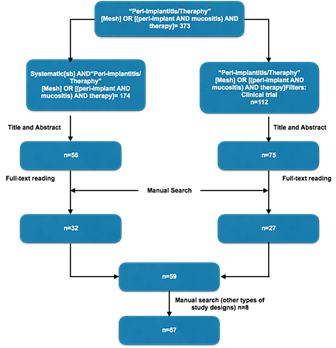
An electronic search of the literature published between 2000 and September 2016 was conducted in the following databases: MEDLINE (PubMed), Cochrane, Embase. A manual search was also conducted in the most important dental implant journals (Table 1).
Table 1 Search Strategy
| “Search peri-implantitis” Search (peri-implant AND mucositis) |
| “Search””Peri-Implantitis/therapy” “[Mesh]” Search (peri-implant AND mucositis) AND therapy[all] |
| “Search””Peri-Implantitis/therapy””[Mesh] Filters: Systematic Reviews” |
| “Search systematic[sb] AND (treatment peri-implantitis)” “Search peri-implantitis Filters: Controlled Clinical Trial” |
| Search (peri-implant AND mucositis) Filters: Systematic Reviews Search (peri-implant AND mucositis) Filters: Clinical Trial |
Scientific search: selection
The SIGN instruments and checklists for data collection were used. Each guideline developer presented their assessment of critical reading and methodological quality to the other authors in a plenary session. Subsequently, a consensus was reached on the relevance of decisions and recommendations for each topic.
RESULTS
The electronic and manual searches for the treatment of PIM and PI yielded 373 articles; after reading the abstracts, 286 articles were obtained. After reading the full-text articles selected in the electronic search and some articles identified through manual search, 59 articles were selected based on the inclusion criteria for the development of this guideline. The authors considered it necessary to include 2 case series, 1 animal study, 2 prospective studies, 1 in vitro study, and 2 literature reviews to determine some good practice points (GPPs). In total, 67 articles were included in this CPG (Figure 1).
Risk of peri-implant mucositis and peri-implantitis: the importance of adherence to supportive periodontal therapy in dental implant patients and frequency determinants
To determine the frequency of maintenance appointments, 13 articles meeting the inclusion criteria were selected. Although one SR reported high survival rates of 98.4%,2 a recent SR reported that implant survival depends on several risk factors, including history of periodontitis and systemic diseases, such as diabetes. Therefore, when planning implant treatment, these factors should be considered for implant survival3 (Level of evidence 2++, Grade of recommendation B).
PIM prevalence was 63.4% among subjects in one SR41 and ranged from 19 to 65% in another SR.42 The PI prevalence rates reported in several SRs were high: 18%,41 22%,42 and 45%.40 Prevalence depends on risk factors: population studied, follow-up time, case definition used, and the unit of analysis. These factors should be known before deciding on an implant therapy to estimate the potential success (Level of evidence 2++, Grade of recommendation B). Patients scheduled to receive implants should know their risk profile to understand the survival probabilities and treatment success based on their individual condition. Patients should be closely monitored clinically and radiographically regarding the status of their implants for the early detection of PIM and PI (Level of evidence 4, Grade of recommendation D; CPG).44
One SR24 reported that the incidence of peri-implant diseases might be minimized with regular peri-implant maintenance therapy and the detection of risk factors contributing to PI (Level of evidence 1+, Grade of recommendation A). One study43 showed that the lack of at least one annual maintenance therapy appointment in patients diagnosed with PIM was associated with an increase in the incidence of PI (Level of evidence 1+, Grade of recommendation A). The frequency of peri-implant maintenance therapy should be established according to a risk profile analysis, as is done for the treatment of periodontal diseases.44 The frequency at which peri-implant maintenance therapy should be performed according to the level of risk has not been established, but it was suggested that the frequency should be lower at a higher risk level. There is a strong recommendation for linking patients to peri-implant maintenance therapy (Level of evidence 1+, Grade of recommendation A).
The SRs showed that patients with a history of periodontal disease have an increased risk of implant failure, with a relative risk (RR) of 1.97 (95% confidence interval [CI] 1.38-2.80), an increased risk of postoperative infections, with a RR of 3.24 (95% CI 1.69-6.21),45 and an increased risk of PI, with a RR of 2.17 (95% CI 1.51-3.12).18 For this reason, periodontitis patients should be treated prior to dental implant therapy (Level of evidence 1++, Grade of recommendation A) and should be given appointments more frequently in maintenance programs (GPP). It has also been reported that patients with aggressive periodontitis have a 4-fold increased risk of developing PI17 (Level of evidence 1-, Grade of recommendation B).
Cigarette smoking and diabetes seem to act as risk factors for PI. One SR found a RR of 2.1 (95% CI 1.34-3.29) for cigarette smoking as a PI risk factor when the analysis was based on the implant; however, when the analysis was based on the patient, there was no association.19 Smokers should be informed about the risk of peri-implant disease prior to implant placement and during supportive periodontal therapy. Efforts should be made to encourage the patient to quit smoking (Level of evidence 1++, Grade of recommendation A). A recent SR evaluated two cross-sectional studies, suggesting a relationship between diabetes and PI.46 Diabetic patients should be referred for metabolic control by a physician and should be routinely asked for laboratory findings confirming their overall health status (Level of evidence 1+, Grade of recommendation A) (GPP). Likewise, conditions such as stress and osteoporosis may modify the host’s immune and inflammatory responses; for this reason, these factors should be evaluated in patients scheduled to receive implants. Occlusal overload has been evaluated as a risk factor for peri-implant diseases in one SR,15 but its effect on bone loss is still controversial. This SR is based on animal studies, suggesting that occlusal overload could affect the implant through an inflammatory reaction (GPP). Similarly, patients who have had signs and symptoms of bruxism or occlusal overload should be examined more frequently due to the possible influence of these conditions on the presence of plaque and inflammatory processes. The frequency of maintenance appointments should always be shorter in these patients than in healthy subjects (GPP).
Available evidence and recommendations for the treatment of peri-implant mucositis
Presently, the standard therapy for PIM remains controversial. Some authors stress the importance of plaque control through mechanical therapy, adjunctive use of antiseptics, and the use of local and systemic antibiotics. In recent years, the use of air polishing devices (with glycine and sodium bicarbonate powders), ozone treatment, probiotics, and sonic toothbrushes has been reported. Eighteen articles meeting the inclusion criteria were selected, which were critically read according to the annexes of the SIGN guidelines.
The findings on PIM indicated that the inflammatory response after 21 days of plaque accumulation was greater than that in the gingiva.47 After 3 months of plaque accumulation, the inflammatory infiltrate was 3-fold higher in PIM than in gingivitis.48-49 It was suggested that anti-infective treatment of PIM is needed for the prevention of complications such as PI (Level of evidence 1+, Grade of recommendation A).
One study reported that the prevalence of PIM was higher (48%) in patients who do not follow a maintenance program,50 and another study51 showed that the prevalence of PIM was lower in patients who follow a periodontal maintenance program (20%). The risk of progression from PIM to PI was higher in patients who do not follow a maintenance program (43.9%) than in patients enrolled in a periodontal maintenance program (18%) (Level of evidence 1+, Grade of recommendation A). However, maintenance programs are not always effective for PIM resolution: 30.5% of cases with PIM resolved after 5 years, and PIM remained in 51.5% of cases43 (Level of evidence 2+, Grade of recommendation C). A recent SR24 noted the importance of individualizing the maintenance program according to the risk factors associated with each patient. The findings show that treatment, history of periodontal disease, and frequency of maintenance appointments influence the incidence of PIM. Patients receiving dental implants should be linked to a strict peri-implant maintenance program to avoid the development of PIM and its progression to PI (Level of evidence 1++, Grade of recommendation A).
One RCT94 compared the efficacy of adjunctive mechanical therapy with probiotic supplements (by either topical application or systemically through tablets) versus mechanical therapy and increased oral hygiene. After 3 months, probiotic supplements did not show any additional benefit of clinical and inflammatory parameters. Further research is recommended before implementing or discarding probiotic treatment in the management of PIM in clinical practice. Another RCT compared the application of 4 treatments, 3 of them with ozone. After 21 days, they found that all ozone treatments were associated with lower PIM incidence compared with the control group.52 Further research is recommended before implementing probiotic supplementation and ozone treatment in clinical practice for PIM prevention and management. In addition, there is a lack of studies demonstrating the efficiency of their implementation to encourage the acquisition of this technology in clinical practice (GPP).
Four RCTs53-56 compared debridement alone versus debridement with chlorhexidine for PIM treatment. All four studies found trends for the improvement of clinical parameters with chlorhexidine as adjunctive therapy, but their results were not statistically significant (p>0.05). In addition, one study57 compared different doses of chlorhexidine and did not find significant differences regarding bleeding on probing (BOP) (p=0.25). The use of chlorhexidine in the short term for the treatment of PIM is not recommended; there are no additional benefits from the clinical point of view, and adverse events may occur, including pigmentation and taste alterations (Level of evidence 1+, Grade of recommendation A). Because of the long-term benefits of chlorhexidine, it should be recommended to patients with certain systemic and psychosocial conditions (e.g., individuals with disabilities, Alzheimer’s disease patients, hospitalized patients, and individuals living in senior housing) (GPP).
One RCT95 compared the effect of a 6-month use of triclosan/copolymer dentifrice versus sodium fluoride dentifrice. BOP decreased from 53.8% to 29.1% in the experimental group, whereas BOP increased in the control group. Another RCT58 evaluated the efficacy of triclosan/copolymer in supportive periodontal therapy for PIM prevention and found lower levels of dental plaque accumulation and BOP. The use of an antimicrobial agent incorporated into toothpaste may be useful for the medium and long-term prevention and management of PIM (Level of evidence 1++, Grade of recommendation A). However, there is a lack of studies supporting this recommendation according to the individual risk profile (GPP).
One RCT59 compared non-surgical treatment of PIM with non-surgical treatment plus systemic antibiotics (azithromycin). After 1.3- and 6-month follow-up periods, no statistically significant differences (p=0.16) were found for clinical parameters such as clinical probing depth and bleeding on probing. The current evidence is insufficient to recommend the use of systemic antibiotics in the treatment of PIM (Level of evidence 1+, Grade of recommendation A). There are no additional benefits in terms of clinical parameters, and adverse events such as antibiotic resistance and harmful gastric effects could occur. There is evidence against the use of systemic antibiotics in the treatment of PIM (GPP).
A recent RCT60 evaluated the effectiveness of sonic brushes for plaque removal compared with manual brushes. After a 2-month follow- up, statistically significant differences were found (p=0.043) regarding the reduction of the modified plaque index. However, this study had a reduced sample size and possible conflicts of interest. The evidence is insufficient to recommend the use of these devices as replacements for conventional manual brushes, as there is a lack of studies evaluating their long-term effectiveness in different clinical situations (Level of evidence 1-, Grade of recommendation B).
Another recent RCT61 evaluated the effect of glycine powder air-polishing versus ultrasound in the treatment of PIM. A 1-year follow-up concluded that both treatment modalities effectively reduced plaque accumulation and peri-implant mucosal bleeding. However, the evidence is insufficient to recommend its use in the treatment of PIM (Level of evidence 1+, Grade of recommendation B). Further studies are required to evaluate the efficiency of glycine powder air-polishing according to the individual risk profile (GPP).
Available evidence and recommendations of different treatment options for peri-implantitis
A wide variety of non-surgical and surgical treatments have been proposed for the treatment of peri-implant diseases. A total of 37 articles were included in this CPG to assess PI treatment. One meta-analysis62 included RCTs and non-randomized clinical trials (NRCTs) to compare non-surgical versus surgical treatments. The proposed non-surgical treatments are scaling the implant surface with plastic or titanium curettes,63 laser,64 and air-polishing devices.65 Only one SR of RCTs compares different non-surgical treatments. The differences reported in these two articles should be carefully considered because of the small discrepancy found in the results with both treatments and wide confidence intervals, suggesting heterogeneity of the results in the meta-analysis. The SRs identified for the recommendations of this guideline33,62,66-69 detected weaknesses in the existing literature for the treatment of PI. Some of the limitations of the evidence are the short follow-up period of the studies and the lack of the implementation of measures with clinical impact.34
Two SRs found that mechanical treatment with some type of adjunctive therapy pro- vides better results than debridement alone for the treatment of PI. In one SR, better results were found with antibiotic therapy70 (Level of evidence 1+, Grade of recommen- dation A), and in another, it was reported that adjunctive therapies (local antibiotics, glycine powder air-polishing, and laser) provided a better response regarding BOP26 (Level of evidence 1+, Grade of recommendation A). Two studies compared the efficacy of piezoelectric ultrasonic oscillating systems versus manual instrumentation with titanium or carbon curettes. There were no significant differences between the two treatments regarding PPD (p=0.30), but both reported improvements in inflammatory parameters63,71 (Level of evidence 1+, Grade of recommendation A). There is conditional evidence supporting the use of manual instrumentation with curettes and/ or ultrasound in the treatment of PI. However, the use of adjunctive therapies is recommended to improve clinical outcomes (GPP).
A recent SR30 evaluated the efficacy of different types of laser for the treatment of PI. Two laser systems were mainly evaluated for the treatment of PI: an erbium:yttrium-aluminum-garnet laser (Er:YAG) (2,940 nm) and photodynamic therapy using a diode laser (660 nm) with a phenothiazine chloride photosensitizer. There are no studies in humans evaluating the effect of Nd-YAG in cases of comparing PDT versus the use of minocycline microspheres after a 12-month follow-up (Level of evidence 1+, Grade of recommen- PI. One RCT72 evaluated the use of CO2 laser in humans. There were no differences in the long-term use of CO2 in conjunction with soft tissue resection (p<0.005) compared with air-polishing devices for decontamination of the implant (Level of evidence 1+, Grade of recommendation A). Of all laser treatments, the Er:YAG laser has been the most thoroughly studied. One RCT64 evaluated the efficacy of the Er:YAG laser compared with mechanical debridement with plastic curettes alone or with chlorhexidine as adjunctive therapy. After 6 months, BOP decreased from 83% to 31% in the laser group and from 80% to 58% in the adjunctive chlorhexidine therapy group; the difference was statistically significant (p<0.001). There were no differences in attachment level changes between the three treatments. Laser treatments (Er:YAG) had a positive impact on BOP compared with other treatments (Level of evidence 1-, Grade of recommendation B). Another RCT73 compared the effectiveness of air-polishing versus laser in patients with severe PI. Both groups improved clinical parameters at 6 months, but there were no statistically significant differences between them (p=0.84) (Level of evidence 1+, Grade of recommendation A). The efficacies of mechanical debridement with titanium curettes and glycine powder air-polishing and adjunctive therapy-either photodynamic therapy (PDT) or minocycline microspheres-were evaluated in one clinical trial at 6 and 12 months.74 After 6 months, inflammation resolved in 15% of the implants in the control group and 30% in the test group, but there was no statistical significance (p=0.16). This study showed that in early PI cases, there was no difference in the resolution of inflammation when comparing PDT versus the use of minocycline microspheres after a 12-month follow-up (Level of evidence 1+, Grade of recommendation A).74-75 Treatment with an Er:YAG laser has no apparent risk of harm to the patient (GPP).76-77 The balance between using either an Er:YAG laser and mechanical therapy with curettes and/or irrigations or glycine powder air-polishing (prophy-jet) affects the usefulness of this technology based on the preferences of the professional. More research is required for a meta-analysis and its evaluation in different clinical situations. More studies (RCTs with low risk of bias) are needed to support the recommendation for the routine use of this technology in the treatment of PI. The equipment is costly, and its benefit is not yet perceived by either the patient or the clinician (GPP). There is no evidence to evaluate the results of different laser treatments in regenerative procedures (GPP).78-79
The evidence for the use of an air-polishing device (prophy-jet) for non-surgical and surgical treatment of PI is limited. The most commonly used air-polishing powders contain glycine (amino acid) and sodium bicarbonate. Air-polishing in vitro models are promising. In vitro, there is evidence of implant surface cleaning from 85 to 100% (GPP). There is evidence of slight surface alterations, but in vivo evidence is weak due to the application methods used (with or without flap), among other factors. One SR, suggests that smooth-surface implants should be treated with rubber cups and non-metallic instruments and rough-surface implants with air-polishing and non-metallic instruments. However, the clinical impact of these decisions is not known (GPP).80 Two RCTs65,81 did not find significant differences (p>0.05) when comparing air-polishing with Er:YAG laser treatment or non-surgical mechanical therapy using chlorhexidine in terms of the clinical attachment level. However, air-polishing was associated with a greater reduction in BOP compared with the local application of chlorhexidine (Level of evidence 1++, Grade of recommendation A). In vitro studies82 have shown better implant surface cleaning with air-polishing, mainly in large peri-implant defects, but the clinical significance of this procedure has not yet been established. There is a consensus among the authors about the difficulty of achieving complete implant surface decontamination. There is conditional evidence supporting the application of air-polishing to achieve partial implant surface decontamination. There is a lack of longitudinal studies assessing the stability of clinical outcomes after long-term application (GPP). In addition, the use of air-polishing could cause an adverse effect (subcutaneous emphysema) after subgingival application (GPP).83
Three adjunctive antibiotics (metronidazole, doxycycline, and minocycline) for mechanical therapy have been evaluated by RCTs. One Bayesian meta-analysis showed a non-significant trend in the reduction of periodontal probing depth (PPD) with the use of antibiotics compared with mechanical therapy alone: 0.490 mm (95% CI -0.647 to 1.252). A non-significant trend in the reduction of PPD was also found with the use of mechanical therapy and PerioChip compared with mechanical therapy alone: 0.400 mm (95% CI -0.843 to 1.629). When debridement and chlorhexidine (control) was compared with debridement and antibiotics, the latter showed greater reduction of PPD: 0.262 mm (95% CI -1.260 to 0.771). The best non-surgical therapeutic approach was the VectorTM system, followed by debridement with adjunctive chlorhexidine (PerioChip) and photodynamic therapy. Debridement alone showed the least favorable results. There is strong evidence supporting the use of adjunctive antibiotics with mechanical debridement in the treatment of some PI cases34 (Level of evidence 1++, Grade of recommendation A).
One of the objectives of PI treatment is the resolution of inflammation; however, it is important to achieve the reconstruction of the peri-implant supporting bone by means of regenerative techniques that apply the concept of guided bone regeneration using bone grafts and membranes.84
There is currently limited evidence from studies comparing the clinical efficacies of different biomaterials and regenerative techniques.68
The following regenerative techniques and biomaterials have been evaluated for the treatment of PI:
Regenerative therapy with nanocrystalline hydroxyapatite
Regenerative therapy with autologous bone
Regenerative therapy with xenograft
Regenerative therapy with allograft
Regenerative therapy with porous titanium granules
Regenerative therapy with beta-tricalcium phosphate
Regenerative therapy with resorbable and non-resorbable membranes
Regenerative therapy versus conventional therapy
A recent SR85 evaluated the changes in clinical and radiographic parameters in non-surgical treatment and surgical resective and regenerative treatment of PI. This study reported that non-surgical treatment reduced BOP and marginal bone loss but did not significantly reduce PPD (p=0.80). In contrast, surgical resective and regenerative treatment showed statistically significant results in the reduction of BOP, marginal bone loss, and PPD (p<0.001) (Level of evidence 1+, Grade of recommendation A). The reduction of marginal bone loss was greater with regenerative therapy [1.703 mm; (95% CI 1.266 to 2.139)] than with conventional surgical therapy [-0.116 mm; (95% CI, -0.433 to 0.201)] for the effective treatment of PI. When the aim of treatment is reducing PPD and marginal bone loss, surgical treatment is indicated for PI more frequently than non-surgical treatment. There is no evidence demonstrating these results in different clinical situations and for individual risk profiles (GPP).
Another recent SR35 evaluated the results of PI treatment with four types of interventions (flap surgery, resective therapy, bone grafting, and guided bone regeneration). The application of membrane graft materials resulted in a greater reduction of clinical probing depth [3.16 mm (CI 95% 2.54 to 3.78 mm)] and increased radiographic bone fill [2.16 mm (95% CI 1.36 to 2.96 mm)] (Level of evidence 2++, Grade of recommendation B). There is conditional evidence recommending the use of bone substitutes and membranes for the regeneration of peri-implant bone defects. More RCTs and SRs of RCTs are required to assess the effectiveness of re- generative treatment with bone substitutes and membranes and their long-term stability (GPP).
To evaluate the effectiveness of different regenerative treatment options, three SRs,35-36,68 six RCTs,71,78,86-89 two case series,90,92 and two longitudinal studies were selected.(91,93 One study longitudinally compared the results of bone augmentation with nanocrystalline hydroxyapatite (Ostim) versus xenograft (Bio-oss) together with resorbable collagen membrane (Bio-Gide). Peri-implant bone defects greater than 3 mm were selected. After 4 years, there were significant improvements in clinical attachment (1.4 mm) and PPD (1.4 mm) for the group that used xenograft (Bio-oss) compared with nanocrystalline hydroxyapatite. There were no differences for changes in gingival recession. The combination of xenograft with resorbable membrane seems to provide better clinical results, possibly due to the chemical stability of the biomaterial, providing a stable bone fill (Level of evidence 3, Grade of recommendation D). There is insufficient evidence to substantiate the use of nanocrystalline hy- droxyapatite. Clinical trials with a larger sample size and low risk of bias are needed to confirm these preliminary results (GPP).
One study86 compared two treatment groups: in the first group, resective surgery, autogenous bone, collagen membrane, and antibiotics were used, while the second group used resective surgery, xenograft, collagen membrane, and antibiotics. After 12 months of evaluation, the xenograft group showed greater radiographic bone fill (1.1 mm) than the autogenous bone group (0.2 mm). Both treatment methods were effective in reducing PPD, BOP, and suppuration without statistically significant differences (p<0.05) (Level of evidence 1-, Grade of recommendation B). There is conditional evidence supporting the use of both biomaterials. There are several factors that can influence treatment outcome, such as the overall condition of the patient, characteristics of the defect, and the method used to decontaminate the implant (GPP).
Another study78 compared implant surface debridement using Er:YAG laser irradiation to plastic curettes after flap surgery, granulation tissue removal, and implantoplasty in patients with advanced PI. Bone augmentation was performed with xenograft and collagen membrane in both groups. Bone loss, BOP, and attachment level were evaluated. After 24 months of evaluation, they found greater bone defect fill and improved clinical parameters in the group treated with plastic curettes (Level of evidence 1+, Grade of recommendation A). There is conditional evidence for the use of an Er:YAG laser with xenograft in the treatment of PI. The long-term stability of clinical outcomes after combined surgical therapy in advanced PI should be evaluated in prospective longitudinal studies (GPP).
One RCT87 compared the results of treatment with porous titanium granules (PTG) versus flap surgery on peri-implant bone defects, and a higher percentage of radiographic bone fill was found in the group of porous titanium granules after 12 months. Although there were significant improvements in clinical parameters with both treatment methods for the different variables studied (p<0.001), no statistically significant differences were observed between the two groups (Level of evidence 1+, Grade of recommendation A). A recent RCT88 found greater statistically significant radiographic bone fill in the PTG group after 12 months (p<0.001); however, there was no statistically significant difference in resolution of PI (p=0.02) (Level of evidence 1+, Grade of recommendation A). There is conditional evidence supporting the use of PTG for the treatment of peri-implant bone defects regarding radiographic bone fill. Longitudinal studies are required to evaluate the long-term stability of bone fill obtained with the application of PTG (GPP).
One study90 evaluated two regenerative approaches: one group received xenografts (Bio-oss/group 1), and the other received allografts (Puros/group 2), both previously hydrated with platelet-derived growth factor (Gem 21) for 5 minutes. The reductions in probing depth were 5.4 and 5.1 mm in groups 1 and 2, respectively, while bone gains were 3.75 mm in group 1 and 3.0 mm in group 2. There is insufficient evidence to support the use of allografts in the treatment of periimplant bone defects (Level of evidence 3, Grade of recommendation D). Clinical outcomes reported in this surgical protocol were based on the clinical experience of the authors. Controlled clinical trials are required to validate this therapeutic approach (GPP).
Another study89 evaluated 41 peri-implant defects to define the outcomes of guided bone regeneration with allograft alone (FG), allograft with non-resorbable membrane (FGM), and allograft with resorbable membrane (FGRM). After three years of follow-up, there were no statistically significant differences in PPD or bone gain (p<0.005). There was a trend to obtain better results with non-resorbable membranes. There is conditional evidence supporting the use of resorbable and non-resorbable membranes in the treatment of peri-implant bone defects. The high rate of exposure of non-resorbable membranes has been shown to affect the periodontal regeneration potential. Self-contained defects may not require the use of a membrane (Level of evidence 1-, Grade of recommendation D). Because application of a membrane is costly, time-consuming, and technique-sensitive, its potential benefits should be carefully considered before its use. Further studies on the different biomaterials or their combination are required, according to the patient’s risk profile and characteristics of the peri-implant defect (GPP).
In one study,72 different bone augmentation techniques were performed for 32 implants. In group 1, beta-tricalcium phosphate (BTP) combined with autologous bone and non-resorbable membrane (Gore-Tex) were used with manual surface decontamination, whereas in group 2, implants were decon- taminated with a CO2 laser prior to the application of BTP. Four months later, the DIB value was lower for the laser group; however, at 5 years, there was no statistically significant difference between the two groups (p<0.005). There is conditional evidence sup- porting the use of BTP in the treatment of PI. No differences have been reported between manual conditioning versus CO2 laser on the implant surface. CO2 laser conditioning prior to regeneration with BTP may be more effective in narrow and deep intrabony defects (Level of evidence 1-, Grade of recommendation B) (GPP).
RECOMMENDATIONS FOR GUIDELINE IMPLEMENTATION
To date, there are no CPGs based on scientific evidence, and CPGs are not structured according to internationally recognized standards for the treatment of peri-implant diseases (PIM and PI). This guideline was developed according to the SIGN criteria. Dental implant patients should be enrolled in a strict periodontal maintenance program, and the frequency of their care should be determined according to their risk factors. The dissemination of clinical practice guidelines that facilitate the prevention and early detection of cases of PIM and PI is required. There is also a lack of orientation for patients and professionals on the prevention of peri-implant diseases and their different treatments.
Future studies should examine the results of the implementation of this CPG in reducing the incidence of PI. In addition, it should be considered that the strict application of this CPG depends on the context in which it is implemented and on the access to certain technologies in different clinical practices.
CONCLUSIONS
Despite the large number of studies on the treatment of PIM and PI, the evidence is still limited for decision-making based on the risk profile. The results obtained in this CPG should be interpreted cautiously. This CPG will be updated every two years or sooner if it is considered pertinent based on the number of studies being published on the topic.