INTRODUCTION
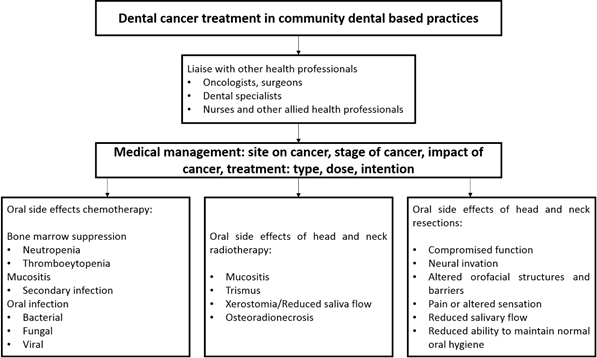
One of the most complicated patient categories in Special Needs Dentistry (SND) centers around cancer management ranging from the illness itself and the management of oral-health related complications. As such, cancer management is often multifaceted involving interventions from a range of disciplines predominately due to the array of complications that can arise as a result of both the illness and its management. Patients with cancer need early dental intervention to avoid the potential risk of systemic and oral complications.1,2 Ideally, oral management should be embedded as part of a multidisciplinary team approach including oncologists, nurses, dentists, social workers, dieticians and other health professionals.2-4
A recent Colombian study which evaluated the oral status in patients who had received radiotherapy to head and neck found that common oral complications in this population included hyposalivation, trismus and periodontal disease.5 This study, which also explored dentists’ involvement in the management of these patients, indicated that dental care was the most common unmet health care need for cancer patients. Dentists played little or no role as part of the multidisciplinary team involved in patient care. The barriers identified cantered around health care delivery including the lack of proper referral pathways, inadequate oral health services and inappropriately trained dentists.5
Dental specialists, predominately Special Needs Dentists, with additional training and experience are required to manage the oral health of individuals with cancer and indeed those with complex healthcare needs.6,7 However, training in SND in Colombia, unlike other specialties, is not offered in tertiary specialist training facilities since it is not recognized as a dental specialty (8 Additional training is available in other South American countries such as a postgraduate degree offered by the Universidad de Buenos Aires.9 Hence those in Colombia must seek entrance to such training programs there or travel abroad to upgrade their skill base. The main drawback of limited options for additional training results is a workforce inexperienced in managing these individuals, in particular the cancer population.5 Regardless of the steps being taken to address this shortage, which will be of benefit in the long term, general dental training needs to be improved to meet the needs of the patients today.5 It is not surprising that Colombian general dentists may be unwilling or uncertain about how to engage with multidisciplinary teams or lack a comprehensive understanding of managing cancer patients.7,10 While there has been some useful information about altering oral care, particularly for those with head and neck cancers, an established process for the oral health care management for this population related to our specific clinical setting is lacking.5 The purpose of this paper is to provide a protocol for the oral health care management for patients with cancer that attend community-based dental practices in Colombia and other countries that lack access to specialists in special needs dentistry.
Dental implications for cancer patients and management
Before initiating any dental treatment, a risk assessment should be completed due to potential complications associated with cancer diagnoses and their management (Figure 1).11 For example, the medical status of some patients, such as those with leukemia, multiple myeloma and Hodgkin’s lymphoma, may interfere with the priority or timing of dental treatment.4,12
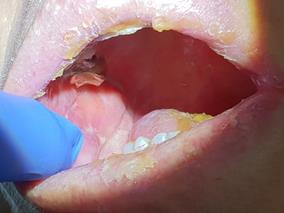
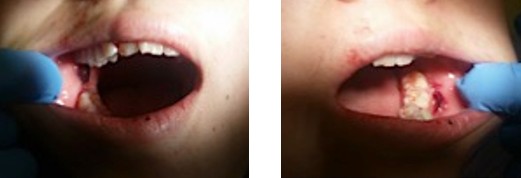
Intraoral conditions, such as gingival hyperplasia in a patient with leukaemia, may be the first clinical manifestations of cancer which may first present to a general dentist. Dentists should be aware of any suspicious changes in patients’ oral health beyond just the teeth.13 Thrombocytopenia manifests as petechiae (small dot like reddening of the mucosa) and ecchymosis (bleeding of the oral mucosa).4 Likewise, non-healing wounds and oral ulcerations can be the result of granulocytopenia (only detected via a blood test) (Figure 2).12 These should prompt the dentist to refer the patient to a general medical practitioner for further management and investigation.
Source: by the authors
Figure 2 Patient whose first presentation of acute myeloid leukemia was bleeding ulcers.
Cancer patients also commonly present with anemia, leukopenia (white cell count <4400/uL) or thrombocytopenia (platelet count < 150,000/uL) due to the effects on blood cell production by either the malignancy or cancer therapy (e.g. multiple myeloma and leukemia).12 These are important considerations because neutropenia (a decrease in the number of circulating neutrophils below 1000 cells/μL) can predispose patients to life threatening complications such as infection including oral infections.12,14 In addition, infection can lead to other complications including dehydration and malnutrition, which may necessitate cessation of cancer therapy thereby affecting patient survivorship.15
Thrombocytopenia may increase bleeding tendency and impact dental treatment including extractions. Extractions can be performed if the platelet count is above 50,000/uL. It is imperative to follow hemostasis protocols, such as the use of hemostatic agents in the socket and suturing to achieve primary closure.16 Furthermore, platelet transfusions are indicated for patients with platelet count below 50,000/uL before extractions or dentoalveolar surgery, and regional blocks avoided with counts below 30,000/uL due to the risk of hematoma.12 The management of these patients requires close collaboration with the treating medical team and potential hospital admission for dental management if possible.
The organ affected by the primary tumor also requires consideration. For example, liver cancer can decrease the production of clotting factors, leading to an increased bleeding tendency.17 Consequently, liver function tests and coagulation profiles, undertaken by referring medical clinicians or if possible the patient’s medical practitioner should be considered before undertaking any invasive dental treatment.16
Cancer pain is also commonly reported and could be the result of direct tumor involvement (such as impingement on nerves and invasion of tumor into skin, muscles and bone), cancer-induced syndrome (e.g. peripheral neuropathy) or a consequence of diagnostic and therapeutic procedures such as chemotherapy, radiotherapy or surgery.18,19 Pain arising from mucositis can interfere with oral intake leading to adverse outcomes such as weight loss and dehydration.20,21 Dentists, in consultation with the treating physician, should evaluate and treat cancer pain when it has the potential to compromise the oral cavity.22
Other symptoms associated with chemotherapy or radiotherapy include nausea, vomiting and fatigue which also impact on oral health and quality of life.23-25 These individuals may neglect oral care resulting in an increased risk of infection not to mention deleterious changes to the oral structures.26 This, coupled with issues around neutropenia, can cause serious medical complications such as sepsis.27 Vomiting can also alter oral pH predisposing to erosion and subsequent oral pain or caries risk.27-29
Cardiac disorders can also be complicated by cancer therapy.30 Two important considerations when providing dental treatment should be to eliminate pain and minimize anxiety.31 Appointments should be scheduled in the morning when the patient is well rested. Adequate pain control should be offered during treatment and postoperatively. Procedure time should also be kept to a minimum to reduce stress and fatigue, restlessness, sweating, and discomfort.31,32
Metastatic bone disease has significant dental implications with respect to antiresorptive medications such as bisphosphonates and Denosumab.33 These agents, coupled with other factors such as poor oral health, dental extractions, age, smoking, and poorly fitting oral appliances, place patients at a higher risk of developing medication-related osteonecrosis of the jaw (MRONJ).34 Pain, eating discomfort, self-consciousness, poor diet, interrupted meals, irritability, and decreased life satisfaction can ensue, each with potential oral health implications. Infection and jaw fracture can also result if these lesions are not addressed. Therefore, implementing a prophylactic dental assessment and necessary dental interventions prior to these therapies is necessary to reduce MRONJ risk especially given it is often recalcitrant to treatment and management may involve surgical intervention, antimicrobial mouthwashes and antibiotic therapy.35
Oral complications of chemotherapy and radiotherapy
Acute side effects
1. Oral mucositis
Mucositis may develop as a result of disruptive toxicity from chemotherapy and/or radiotherapy (Figure 3).36-38 There are differences in the cellular events of chemotherapy and radiation-induced mucositis. Mucotoxic chemotherapy may be delivered over just a single brief period or spread out over no more than a few days (e.g. haematopoietic stem cell transplant).39 The symptoms are acute with ulcerations developing typically within one week after initiation of chemotherapy and reaching a peak within 2 weeks.40 Ulcerations may develop in the mouth as well as in mucous membranes lining the digestive tract.39 In oral mucositis, lesions are usually limited to non-keratinized surfaces and commonly affected sites include lateral and ventral tongue, buccal mucosa and soft palate.41 With radiation therapy, the damage is applied in several small daily doses over a period of weeks.39 Mucosal injury usually occur at cumulative radiation doses of about 15Gy.39 In radiation-induced oral mucositis, non-keratinized tissues are more often affected, and the clinical severity is directly proportional to the radiation dose. The lesions are specific to the tissues in the field of radiation.40
Prior to ulceration, a white discoloration of the mucosa can be seen as a result of a transient hyper-keratinization of the epithelium.42 This is followed by thinning of the epithelium due to loss of renewal capacity caused by the indirect damage to epithelial stem cell.39 This results in erythema from increased vascularity. The loss of mucosal integrity then manifests as painful ulcers and confluent pseudomembranous reactions with severe necrosis that can result in treatment cessation due to excruciating pain, risk of infection and reduced quality of life.36,39 The pseudomembranous appearance of the mucosa is the result of fibrinous bacterial exudate.39 Mucositis is a self-limiting condition where the ulcers resolve spontaneously within two to three weeks following the completion of treatment.39,43
Scoring systems are used to document the degree of oral mucositis and to evaluate the effects of intervention measures.44 One of the most common grading system is the World Health Organization grading system.45 This score system assesses mucosal changes, symptoms, and functional impact on the patient, and classifies oral mucositis into five groups:
The risk of oral mucositis and management of symptoms should be discussed with all patients prior to receiving cancer therapy with the importance of good oral hygiene highlighted. The recommended management of patients with oral mucositis includes:
Eliminating infection and irritation and establishing effective oral hygiene practices
Frequent use of mouth rinses (e.g. a teaspoon of salt and sodium bicarbonate in a tumbler of warm water to use as frequently as needed)
Avoiding tobacco, alcohol and carbonated drinks
Soft diet (this will require the input of a dietician) and
Maintaining adequate hydration levels.27
The Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) have published evidence-based clinical practice guidelines for the management oral mucositis.46 The guidelines include options such as growth factors, anti-inflammatory agents, antimicrobials, coating agents, anesthetics and analgesics, laser and other light therapy, cryotherapy, and natural and other miscellaneous agents with their suggested use dependent on the type of cancer treatment received.46 For example, low-level laser therapy has been suggested to be used to control the intensity and pain of mucositis.47,48 Mixed medication mouthwashes consisting of topical coating, anesthetic, and possibly other agents have also been suggested as part of the supportive care but there is inadequate and/or conflicting evidence to support their use.46 Based on clinical experience, topical anesthetic agents (viscous xylocaine) can be applied on oral ulcerations to control pain and to allow patients to eat and do oral hygiene; however, the effects are transient.49,50
2. Oral infections
Oral bacterial, fungal and viral infections are significant causes of morbidity amongst cancer patients.51 These may result from changes to the indigenous microflora due to cancer therapies, myelosuppression, impaired host defense mechanisms, salivary gland dysfunction and poor oral health and hygiene.51,52 In addition, exacerbation of dental caries, periodontal disease and odontogenic infections are also potential issues.52 Routine oral hygiene measures and regular dental examination and care should form part of a preventative regime.51
Fungal infections, such as oral candidiasis, are common in cancer patients.53,54 Chemotherapy, radiotherapy, salivary gland dysfunction and mucosal fragility provide an ideal environment for proliferation by candida species which can be managed by prescribing Nystatin 100,000 IU/mL four times/day for 7-14 days.53-55
Neutropenic patients are also at higher risk of developing viral infections with herpes simplex, one of the most common infections resulting in pain, discomfort, dehydration and malnutrition.56 A major oral complication is the spread of potentially life-threatening infection. Pharmacological management includes oral Acyclovir 400 mg three times/day for 10 days or longer and oral Valacyclovir 500-1000 mg twice/ day for 10 days or longer.56,57
3. Dry mouth
Xerostomia (a subjective feeling of dry mouth) and salivary gland hypofunction (chronic reduction of salivary output due to glandular changes) are both common complications of cancer therapy. Although the terms are often used interchangeably, they represent different manifestations that may or may not be related.58 Quantitative and qualitative salivary changes predispose these patients, especially those irradiated for head and neck cancer, to oral discomfort, taste disturbances, difficulty chewing, and impairment of swallowing and speaking.59 In addition, dry mouth can exacerbate caries risk.60 Saliva stimulants, such as chewing gum, have been recommended for symptomatic treatment. Saliva substitutes are also suggested, for example Biotène® Oral Balance Moisturizing Gel.61,62
4. Taste disturbances
Hypogeusia (no taste) and dysgeusia (altered taste) are relatively common in cancer patients and may be the result of the cancer, its management or damage of cranial nerves.63,64 Management may include treatment of the underlying cause and dietary therapy.63
Late side effects
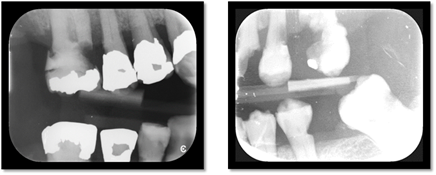
5. Radiation caries (Figure 4)
Source: by the authors
Figure 4 Bitewings radiographs showing evidence of caries in a patient exposed to radiation to head and neck
Radiation caries is mainly attributed to radiation-induced damage to the salivary glands and subsequent reduced saliva production; however, there may be other contributing factors.65 For example, weight loss can be an issue during cancer treatment resulting in patients being recommended to eat frequent small meals with emphasis on high caloric foods. Patients are also supplemented with liquids containing refined carbohydrates that predispose sugar adhesion to dental surfaces.66 Patients may find it difficult to brush between feeding due to the increased frequency of meals which may be further complicated by pain associated with mucositis causing increased plaque accumulation. Patients may also place more importance on other treatment rather than dental care which results in long delays between oral intake and oral hygiene.65,66 Furthermore, it has been demonstrated an increase in cariogenic oral bacteria, including Streptococcus and Lactobacillus species, in patients with head and neck cancer receiving radiation.67 This can lead to progressive destruction of tooth structure due to the acidogenic-aciduric properties of these microorganisms.68
During and after radiotherapy treatment, it is important to establish good oral health habits. Brushing 2-4 times daily with a soft-bristled toothbrush and a high fluoride content toothpaste such as Colgate® PreviDent® 5000 Plus. Flossing is also of paramount importance to oral hygiene once the mucosal status permits this.69,70 Custom carrier trays for application of fluoride are indicated.69 Antibacterial mouthwashes, such as chlorhexidine solutions (0.12%-0.2%), can be used to control plaque accumulation.71 Sugar-free gums containing xylitol can be used to stimulate saliva with the aim of increasing buffering capacity and sugar clearance.72
Frequent follow-up appointments are recommended after completion of radiotherapy.69 Caries removal and restorations should not be delayed.69 Restoration of these teeth may prove to be difficult due to the extent of caries and especially for lesions located cervically.69 Material selection can be also difficult due the challenging oral environment found in irradiated patients.69 The ideal dental material should have good adhesive properties, assist with prevention of secondary caries, and withstand the challenges of a dry and acidic oral environment. Fluoride-releasing materials (e.g. glass ionomer cements) have proven to be effective in the management of caries in irradiated patients although erosion of the material remains a concern.69,73
6. Trismus
Trismus refers to restricted mouth opening (<35mm, measured between the central incisors) that may occur as a direct result of cancer invading the temporomandibular joint or masticatory muscles, or as a consequence of tissue fibrosis from radiotherapy.74,75 Quality of life may subsequently be affected by impairment of communication, oral hygiene and dental care.76 At risk patients should be informed and preventive measures such as exercise therapy, instigated.76 For example, use of wooden tongue depressors to improve both vertical and horizontal jaw motion. It should be initiated as soon as possible following radiation in at risk patients and consists of holding the depressors between the teeth for 30 seconds 6 to 10 times per day and gradually inserting a new tongue depressor in increments of approximately 2 mm to enhance opening.76,77
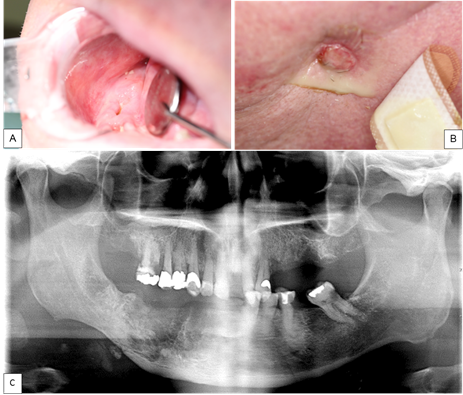
7. Osteoradionecrosis. (Figure 5)
Source: by the authors
Figure 5 A. Intraoral imagen showing an area of exposed necrotic bone 5 months post extraction of teeth 44, 45, 46, and 47. B. Extraoral imagen showing a cutaneous sinus tract on the right side of the mandible. C. OPG showing evidence ORN of the mandible in the right posterior region with a pathological fracture.
Radiotherapy to the orofacial structures places patients at higher risk of developing osteoradionecrosis (ORN), not to be confused with MRONJ. Prevalence rate ranges from 5% to 15%.78 The incidence of ORN is relatively low (5.5%) but management can prove difficult.79,80 ORN is one of the most severe chronic side effects of radiotherapy, being more common in the mandible and is rare where radiation doses fall below 60 Gy.81-83 The pathophysiology remains unclear but proposed to result from defective osteogenesis due to radiation-induced fibrosis.84 It has been also reported that radiation can lead to an imbalance between bone resorption and bone formation due to the damage of osteoblast and increased activity of osteoclasts.85 These changes appear to develop earlier than vascular alterations.86 The main clinical feature is an area of exposed bone following radiotherapy that may occur either after a traumatic event (e.g. dental extraction) or spontaneously in the absence of malignancy in the region.75
Prevention is paramount because its progression may lead to complications, including pathological fractures and local or systemic infection.87,88 Pre-treatment dental assessments include the removal of teeth with poor prognosis (non-restorable teeth with caries, severe periodontal disease, or with latent infections) prior to radiotherapy (preferably at least 3 weeks before to allow healing).27,88,89Patients should be reviewed closely to ensure healing (gingival closure of extraction sites) and subsequently followed regularly (every 1-2 weeks) during radiotherapy. Thereafter, monthly reviews should be performed in the first six months to assist with oral rehabilitation, including treating any caries or new dental conditions, and monitoring of oral hygiene.88
If a dental extraction is required after head and neck radiotherapy, the patient should be referred to an oral surgeon. Treatment of ORN remains difficult and controversial but all suspected cases should be referred for specialist management.88,90 Adjunctive treatments that may be considered include conservative measures, sequestrectomy, hyperbaric oxygen therapy and PENTOCLO treatment (pentoxifylline combined with vitamin E).80,88,90
Table 1 presents a summary of oral complications of cancer treatment and management.
Table 1 Oral complications of cancer treatment, clinical presentation and management12,51,69
| Oral manifestation | Clinical presentation | Management |
| Oral mucositis | Erythema of the mucosa Patchy or confluent ulcerations, sometimes covered by a pseudomembrane Tissue necrosis Bleeding with minor trauma | Elimination of infection and irritation Establish effective oral hygiene practices Frequent use of mouth rinses Advise patients to avoid tobacco, alcohol and carbonated drinks Soft diet Maintaining adequate hydration levels |
| Oral infections | ||
| - Bacterial e.g. odontogenic deep space infection | Facial swelling Pain Fever | In immunocompromised hosts: broad spectrum antimicrobial should be prescribed e.g. cefotaxime 2 g IV q 6 hour |
| - Fungal e.g. pseudomembranous candidiasis | Pseudomembranous patches Mucosal erythema | Nystatin 100,000 IU/mL four times/day for 7-14 days |
| - Viral e.g. herpes simplex virus | Vesicles Small crops of ulcers or more florid reaction Cold sore | Acyclovir 400 mg three times/day for 10 days or longer Oral Valacyclovir 500-1000 mg twice/day for 10 days or longer |
| Salivary gland dysfunction | Xerostomia Oral discomfort Difficulty chewing and swallowing Altered taste Halitosis Oral infection | Symptomatic treatment Saliva stimulants: chewing gum Saliva substitutes: Biotène® Oral Balance Moisturizing Gel |
| Taste disturbances | Unpleasant taste of food | Treatment of the underlying cause Dietary therapy |
| Radiation caries - Type 1 | Most common Affects the cervical aspect of the teeth extending to cementoenamel junction Circumferential decay develops often resulting in crown amputation - Type 2 Areas of demineralization on all dental surfaces Generalized erosion Worn out occlusal and incisal surfaces - Type 3 Least common pattern Color changes in the dentin (Crown becomes dark brown-black) Occlusal and incisal wear | Establish good oral health habits Brushing 2-4 times daily with a soft-bristled toothbrush and a high fluoride content toothpaste Custom carrier trays for application of fluoride Caries removal and teeth restorations should not be delayed |
| Trismus | Restricted mouth opening (<35mm, measured between the central incisors) | Exercise therapy Stretching the mouth using wooden tongue depressors |
| Osteoradionecrosis | Area of exposed bone following radiotherapy Possible pain or secondary infection, May progress to formation of a sequestrum, cutaneous fistula and/or pathological fracture | Management remains difficult Refer patient to an oral surgeon |
Source: by the authors
Treatment planning modifications
General dental practitioners can play an important role in managing cancer patients in all three phases of multidisciplinary management: pre-treatment evaluation and preparation, during treatment and post-treatment.27 In doing so, dentists need to appreciate that there are often challenges faced beyond dental care. Therefore, collaboration with and/or referral to specialists with sufficient oral health and non-oral health training and experience, such as oral surgeons and stomatologists, is beneficial.4,7 In addition, collaboration outside of traditional oral health physicians should also be considered.
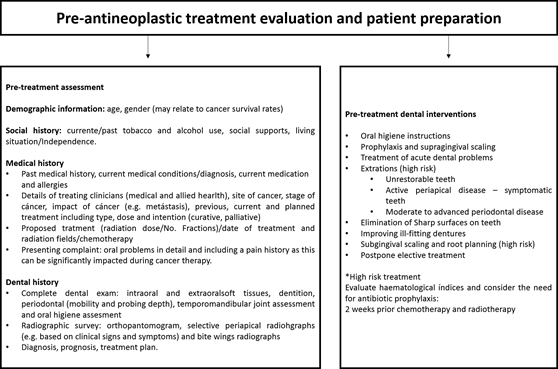
Pre-antineoplastic treatment evaluation and patient preparation
As mentioned previously, the objectives of early intervention include identifying and eliminating sources of infection, identifying oral manifestations of the cancer and minimizing and treating oral sequelae that can arise as a result of antineoplastic therapy (Figure 6).27,63
A comprehensive dental examination should include a record of the dentition and assessment of dentures.91 A panoramic radiograph plus selective intraoral films should be used to determine the presence of periapical abnormalities, dental caries and periodontal disease.92 Full mouth periodontal charting at baseline is also recommended where possible. This should, however, be avoided in patients with platelets counts of less than ³20,000 cells/μL27,93,94 or when neutrophil counts are less than 1,500 cell/mm3.4,95 At this stage, it is important to promote meticulous oral hygiene.93 Commonly used preventive measures are described in Table 2.27,96
Table 2 Preventive measures to maintain oral health in patients with cancer
| - Brush teeth twice daily with high concentrated fluoride toothpaste, for example Colgate® PreviDent® 5000 Plus |
| An ultra-soft or a soft toothbrush is preferred but brushing with sponge brushes is an option in patients with friable mucosa |
| Use products with remineralization properties, for example MI Paste Family - GC America |
| - Chlorhexidine gluconate 0.2% mouth rinses may be recommended (preferably alcohol-free) |
Source: by the authors
Communication with the multidisciplinary cancer care team is essential to discuss the patient’s medical and hematological status.71 Ideally, dental treatment should be tailored to the patient’s needs involving a shared care approach between the dental oncology service and the primary care dentist.71 In some circumstances, patients may receive all treatment from their general dental practitioner.5 Dental treatment should be directed towards acute dental problems, foci of infection, non-restorable teeth, sharp surfaces on teeth and ill-fitting dentures with the frequency of visits determined by each individual’s needs.27,71,97
Dental procedures in the pre-treatment phase should also include oral hygiene instructions, simple restorations for adequacy of the oral environment, prophylaxis and supragingival scaling.4 Elective treatment should be postponed until the patient is medically stable without marked neutropenia or thrombocytopenia.98 The need for antibiotic prophylaxis should also be discussed with the patient’s medical (and allied health) team.4
Scaling, extractions, and endodontic treatment, including retreatment, all carry potential risks in this cohort and their need should be discussed within the multidisciplinary team.4 Dental extractions and periodontal treatment should be completed at least 14 days before cancer treatment commences to allow adequate healing.51 In certain circumstances, delays may be necessary (e.g. where tooth extractions are required prior to head and neck radiotherapy).99 Likewise, patients with hematological cancer require urgent elimination of sources of infection to prevent risk of febrile neutropenia.4
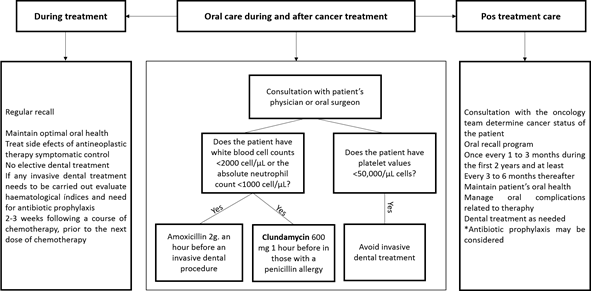
Oral health care during treatment.( Figure 7 )
Dentists should regularly monitor the oral cavity throughout cancer treatment to ensure optimal oral health and manage any side effects of antineoplastic therapy.4 Depending on the cancer therapy planned, it may be possible to complete dental treatment between chemotherapy cycles.4 If invasive dental treatment is required, evaluate hematological indices and the need for antibiotic prophylaxis in conjunction with the patient’s medical team (Figure 4).4 A patient’s blood cells will reach their lowest number (nadir) 7 to 14 days after initiation of chemotherapy. Surgical dental procedures can be performed after the neutrophil count has begun to rise from the nadir.12
Post treatment care
Consultation with the oncology team is also recommended post-cancer therapy (Figure 7).27 Patients should be placed on an oral recall program (every 1-3 months during the first two years, at least 3-6 monthly thereafter).27 The aim is to maintain oral health and detect and manage oral complications related to therapy or cancer reoccurrence. All dental procedures can be performed post-chemotherapy as long as hematological indices are normal.4 Regarding radiotherapy to head and neck, dentists should always remember that patients are at lifetime risk of osteoradionecrosis of the jaw.83
CONCLUSION
To safely provide dental treatment, dentists should be aware of the pathophysiology of cancer and the spectrum of complications associated with the disease and therapies.
Dental management may be preventive, curative or palliative. The dentist’s role within the multidisciplinary team is to ensure coordination of dental care and prioritize oral care appropriate to the patient’s medical needs and within their scope of clinical competency.