Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.17 no.3 Medellín Sept./Dec. 2010
BIOTECHNOLOGY
PRODUCTION OF 2-PHENYLETHANOL IN THE BIOTRANSFORMATION OF CINNAMYL ALCOHOL BY THE PLANT PATHOGENIC FUNGUS Colletotrichum acutatum
PRODUCCIÓN DE 2-FENILETANOL EN LA BIOTRANSFORMACIÓN DE ALCOHOL CINAMÍLICO MEDIANTE EL HONGO FITOPATOGÉNICO Colletotrichum acutatum
Rodrigo VELASCO B.1; Jesús H. GIL G.1,2; Carlos M. GARCÍA P.1, Diego L. DURANGO R.3
1 Grupo de Química de los Productos Naturales y los Alimentos. Escuela de Química. Facultad de Ciencias. Universidad Nacional de Colombia. Calle 59ª 63-020 Autopista Norte, AA 3840. Medellín, Colombia.
2 Departamento de Ingeniería Agrícola y Alimentos. Facultad de Ciencias Agropecuarias. Universidad Nacional de Colombia. Sede, Medellín. Medellín, Colombia.
3 Grupo de Química de los Productos Naturales y los Alimentos. Escuela de Química. Facultad de Ciencias. Universidad Nacional de Colombia. Calle 59ª 63-020 Autopista Norte, AA 3840. Medellín, Colombia. dldurango@unal.edu.co.
ABSTRACT
Biocatalytic processes may offer a cheaper alternative to natural production of flavours. The biotransformation of cinnamyl alcohol is investigated using the plant pathogenic fungus Colletotrichum acutatum as a biocatalyst. Results show that substrate is converted to 3-phenyl-1-propanol, 1-phenyl-1,3- propanediol, 2-phenylethanol, 1-phenyl-1,2-ethanediol, 3-phenyl propyl acetate, and hydrocinnamic acid. The structures of the metabolic products are elucidated on the basis of their spectral data. 2-phenylethanol has a sweet, floral odor and a wide variety of applications, especially, for the perfume and food industries. A time-course experiment demonstrates that 2-phenylethanol appeared after 120 hours, reaching almost 8% of relative abundance. Additionally, the influence that the culture broth has on the conversion capacity is investigated. It has been discovered that cinnamyl alcohol is converted faster when the substrate is incorporated in a Sabouraud medium; under this condition, 2-phenylethanol is the most common product after 288 hours reaching about 90% of the relative abundance. Biotransformation of cinnamyl alcohol using C. acutatum in a Sabouraud medium can offer a simple and efficient way to obtain 2-phenylethanol with high yield.
Keywords: biotransformation, Colletotrichum, reduction, 2-phenylethanol, antifungal assay.
RESUMEN
Los procesos biocatalíticos pueden ofrecer una alternativa económica para la producción natural de sabores. La biotransformación de alcohol cinamílico es investigada usando el hongo fitopatógeno Colletotrichum acutatum como biocatalizador. Los resultados muestran que el sustrato es convertido en 3-fenil-1-propanol, 1-fenil-1,3-propanodiol, 2-feniletanol, 1-fenil-1,2-etanodiol, 3-fenil propil acetato, y ácido hidrocinámico. Las estructuras de los productos metabólicos son elucidados con base en la información espectroscópica. El 2-feniletanol tiene un olor floral suave y una variedad de aplicaciones, especialmente, en perfumería y alimentos. Un estudio en el curso del tiempo demuestra que el 2-feniletanol aparece después de 120 horas y alcanza casi 8% de la abundancia relativa. Adicionalmente, se investiga la influencia del medio de cultivo en la capacidad de conversión. Se encuentra que el alcohol cinamílico es convertido más rápido cuando el sustrato es incorporado en medio Sabouraud; bajo esta condición, el 2-feniletanol es el producto mayoritario después de 288 horas alcanzando cerca del 90% de la abundancia relativa. La biotransformación de alcohol cinamílico mediante C. acutatum en medio Sabouraud puede ofrecer una alternativa simple y eficiente de obtener 2-feniletanol con alto rendimiento.
Palabras clave: biotransformación, Colletotrichum, reducción, 2-feniletanol, actividad antifúngica.
INTRODUCTION
2-Phenylethanol (2-PE), whose chemical structure is presented in figure 1, is a colorless liquid possessing a faint but lasting rose petal odor, and occurs naturally in the essential oils of many flowers and plants, such as hyacinths, jasmines, narcissi and lilies (1). The rose fragrance is highly desired, making 2-PE one of the most commercially used fragrance chemicals in perfumes, soaps, and detergents (2). In addition, 2-PE has bacteriostatic and antifungal properties (3); which is why this alcohol is used in the preparation of antiseptic creams and deodorants. It is also extensively used in the formulation of various cosmetics, especially in hair shampoos and hair dyes, to improve the texture and quality of hair (4). Moreover, it is used in the composition of flavors of food products, such as soft drinks, cookies, chewing gum, pudding, and more (5).
Currently, most of the 2-PE present in the market is chemically synthesized, via a Friedel– Craft reaction of ethylene oxide with benzene in the presence of molar quantities of aluminum chloride, by catalytic reduction of styrene oxide with Raney nickel as a catalyst, or as a byproduct of the production of propylene oxide (6). All these methods involve toxic reagents and harsh conditions, thereby creating a byproduct that reduces the quality of the final 2-PE. Removal of the undesired contaminants is necessary before marketing the product (7).
In recent years, the consumer preference for natural products has stimulated the search of alternative processes for producing natural flavors. Natural 2-PE can be extracted from rose shrubs and other plants but, due to the rarity of the raw materials, natural 2-PE costs approximately 250 – 300 times more than its chemically produced counterpart (8). It is very improbable that the supply of natural 2-PE from plants can meet the market demand. The progressively increasing market of natural flavors has forced suppliers to search for alternative sources. An advantageous way to obtain natural flavors is the use of biotechnological processes involving microorganisms which would be independent of restrictions related to plant supply (weather, diseases, and trade restrictions). Fungi are known for their high ability to synthesize aromatic flavors (9). Some species such as Polyporus tuberaster (10), Ischnoderma benzoinum (11), Aspergillus niger (12) and Kluyveromyces marxianus (13) have been particularly studied for the bioconversion of appropriate natural precursors (such as L-phenylalanine) into 2-PE. Consequently, a biotechnological means may provide highly pure natural 2-PE through an environmentally friendly process (5).
Colletotrichum is one of the most relevant plant pathogens worldwide, causing the economy affecting disease anthracnose in a wide range of hosts, including cereals, legumes, vegetables, perennial crops and tree fruits (14). Several species of the genus Colletotrichum, like C. gloeosporioides (Penz.) Penz & Sacc. (teleomorph Glomerella cingulata), C. lini and C. musae (Berkeley et Curtis) von Arx, have been used in biotransformations (15-17). However, the ability of C. acutatum to produce microbial transformations has been scarcely investigated, although this species was recently shown being involved in bioconversion processes to produce 2-PE from acetophenone (18). This work reports the study of the bioconversion of cinnamyl alcohol (A), a natural substrate found in essential oils of narcissus, lilac and cinnamon, into 2-phenylethanol by C. acutatum in different culture media.
MATERIALS Y METHODS
Analytical methods
A thin layer chromatography (TLC) was made on precoated plates (Si 60 F254, 0.25 mm, Merk). Mixtures of n-hexane:EtOAc were the mobile phase. Compounds were visualized under UV radiation at 254 and 365 nm, and by aspersion with AcOH:H2SO4:H2O (143:28:30) followed by brief heating. Column chromatography (CC) used silice gel 60 (0.040-0.063 mm; Merck) and Sephadex LH-20. Gas chromatography (GC) was performed on a Hewlett-Packard 6890 (Agilent Technologies) gas chromatograph coupled with a HP 5973 MSD (Mass selective detector-Quadrupole type). A HP-5 column (30 m x 0.25 mm i.d.; coating thickness 0.25 μm) was used. Chromatographic conditions were as follows: column temperature, 50-250°C at 10°C/min and maintained for five minutes; injector temperature, 150°C; detector temperature, 280°C; carrier gas, N2 at 1 mL/min. The relative composition of the individual constituent was determined from the peaks average area. EI-MS measurements were obtained using gas chromatography-mass spectrometry (GCMS). Chromatographic conditions were the same as described above. Substances were identified by comparison of their mass spectra with those of reference substances, and by comparison with the NIST 2002 Mass Spectral Library. Infrared spectra (IR) were performed on a Perkin Elmer Paragon 1000. NMR (Nuclear Magnetic Resonance) spectra were measured on a Bruker AMX 300 NMR spectrometer (1H NMR, 300.12 MHz; 13C NMR, 75.42 MHz). Chemical shifts, δ, are expressed in ppm units downfield from TMS and coupling constant J are in hertz (Hz).
Biological and chemical materials
The strain of C. acutatum, kindly provided by the Laboratory of Phytopathology (Universidad Nacional de Colombia, Medellín), was isolated from diseased Solanum betacea cav. Sendt (tamarillo) fruits. Also, the microorganism was morphologically and molecularly characterized by Dr. Afanador-Kafuri (19). The fungus was maintained in a Potato Dextrose Agar (PDA) medium at 24±2°C, and monthly subcultured in Petri dishes. To evaluate the antifungal activity, previously sterilized 15 cm diameter Petri dishes were inoculated with 1 mL of a spore suspension of the fungus. The suspension was uniformly spread over the medium using a bent glass rod. Then, the inoculated medium was incubated at 25°C for 48 h. A 5 mm diameter mycelial disc was used for antifungal testing. The substrate for biotransformation, cinnamyl alcohol (A), and compounds 2-phenylethanol (2-PE), 3-phenyl-1-propanol (B) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Bacto Agar was obtained from Becton, Dickinson and Co (Sparks, MD, USA). Yeast extract was purchased from Oxoid Ltd (Basingstoke, UK). Peptone from casein (pancreatically digested) was purchased from Merck KGaA (Darmstadt, GER).
Antifungal bioassay
In order to investigate the antifungal activity of the cinnamyl alcohol (A) (analytical grade) against the microorganism, the mycelial growth test with PDA was used. Different concentrations (100-600 mg/L) of (A) dissolved in ethanol were diluted in Petri dishes with PDA. Subsequently the fungus was inoculated immediately by placing in the center of each plate a 5 mm diameter of the mycelial mass of the cultivated test fungus, cut with a sterile cork borer from the periphery of growing cultures on PDA plates prepared as described above. Petri dishes with ethanol (2mL/L) were used as a control. Petri dishes were incubated at room temperature and the diameter of the mycelial growth was measured every 24 hours. The incubation was stopped when the mycelial mass of control Petri dishes had almost filled it completely (ca. 240 hours). All concentrations were tested in triplicate. The relative growth inhibition of the treatment, compared to the control, was calculated as a percentage using the following expression:
Inhibition (%) = {1–[radial growth of treatment (mm)/radial growth of control (mm)]}x100
Equation 1.
Preculture of Colletotrichum acutatum
The fungus was inoculated into 1.0 L Erlenmeyer flasks, containing 500 mL of Czapeck-Dox liquid medium (Solution A: Glucose 5%, yeast extract 0.1%; Solution B: K2HPO4 0.5%, NaNO3 0.2%, MgSO4.7H2O 0.05%, FeSO4.7H2O 0.001%). Erlenmeyer f lasks were shaken (reciprocating shaker, 120 rpm; Centricol series 0239, with incubation chamber) at room temperature for 192 hours. Mycelia were harvested by filtration, washed with H2O and employed in the biotransformation and time course experiments.
Preparative biotransformation
The mycelium of C. acutatum was transplanted into four 1.0 L Erlenmeyer flask containing 500 mL of sterilized culture medium and substrate (at 400 ppm). Cultivation was carried out stirring (reciprocating shaker, 120 rpm) at room temperature for 336 hours. After the incubation period, culture medium and mycelia were separated by filtration. Mycelia were discarded and culture medium was used to isolate the metabolic products. Control was carried out in order to verify the presence of similar compounds on the fungi culture (without substrate).
Isolation and identification of products The culture medium was saturated with NaCl and extracted with CH2Cl2 (3x2.0 L). Afterwards, the medium was acidified to pH 2 with 1M HCl, and extracted again with CH2Cl2 (2x2.0 L). Both organic extracts were mixed, dried over anhydrous Na2SO4, and concentrated in vacuo. The crude extract was chromatographed on a silice gel column; elution was performed with a n-hexane-EtOAc gradient system. Several fractions were collected and divided into 6 groups (I-VI) by TLC profiles. Fractions III and IV were fractionated by size-exclusion column chromatography over Sephadex LH-20 (100x2 cm) using n-hexane-CH2Cl2-MeOH (50:25:25, v/v) as eluent to yield metabolic compounds B, C and 2-PE. The structures of the products were determined by MS, IR, 1H and 13C NMR analysis, and comparison with authentic samples and the NIST 2002 computerized mass spectral library.
3-phenyl-1-propanol (B): 1H NMR (CDCl3, 300 MHz): δ 1.85-1.95 (td, 2H, J = 7.7, 6.4), 2.70 (t, 2H, J = 7.7), 3.69 (t, 2H, J = 6.4), 7.20-7.30 (m, 5H); 13C NMR (CDCl3, 75 MHz): δ 32.0 (C3), 34.5 (C2), 62.4 (C1), 125.5 (C4'), 128.2 (C2', 3', 5', 6'), 142.0 (C1'); EI-MS, m/z [rel. int.]: 136 (M+) [21], 118 (M-H2O+) [54], 117 [88], 91 (C7H7 +) [100], 77 (C6H5 +) [26], 65 [25], 51 [17]; IR vmax (CHCl3) cm-1 3350 (-OH), 3030 (=C-H), 2980, 1550 (C=C), 1500 (C=C), 1430, 1390, 1100 (C-O), 950; retention time: 12.15 min.
1-phenyl-1,3-propanediol (C): 1H NMR (CDCl3, 300 MHz): δ 1.93-1.97 (tdd, 2H, J = 8.5, 6.0, 4.0), 3.84 (t, 2H, J = 6.0), 4.95 (dd, 1H, J = 8.5, 4.0), 7.27-7.31 (m, 5H); 13C NMR (CDCl3, 75 MHz): δ 41.1 (C2), 62.0 (C3), 74.9 (C1), 126.3 (C2', 6'), 128.2 (C4'), 129.2 (C3', 5'), 145.0 (C1'); EI-MS, m/z [rel. int.]: 152 (M+) [43], 134 [19], 133 [30], 107 [100], 105 [42], 78 [16], 77 [45]; IR vmax (CHCl3) cm-1 3300 (-OH), 3030 (=C-H), 2980, 1660 (C=C), 1200 (C-O), 1100 (C-O); retention time: 17.24 min.
2-phenylethanol (2-PE): 1H NMR (CDCl3, 300 MHz): δ 2.76 (t, 2H, J = 7.0), 3.69 (t, 2H, J = 7.0), 7.07-7.27 (m, 5H); 13C NMR (CDCl3, 75 MHz): δ 35.2 (C2), 59.4 (C1), 122.3 (C4'), 124.5 (C3', 5'), 125.1 (C2', 6'), 134.9 (C1'); EI-MS, m/z [rel. int.]: 122 (M+) [30], 104 (M-H2O+) [5], 91 (C7H7 +) [100]; IR νmax (CHCl3) cm-1: 3400 (-OH), 3030, 2950, 1600 (C=C), 1493, 1454, 1050; retention time: 9.30 min.
Additionally, compounds (D) and (E) were identified by comparison of mass spectra and GC retention times with those of authentic reference standards (18, 20).
1-phenyl-1,2-ethanediol (D): 1H NMR (CDCl3, 300 MHz): δ 3.75-3.60 (m, 2H), 4.81 (m, 1H), 7.26 (m, 5H); 13C NMR (CDCl3, 75 MHz): δ 68.5 (C1), 75.1 (C2), 126.5 (C2', 6'), 128.4 (C4'), 128.9 (C3', 5'), 141.0 (C1'); EI-MS, m/z [rel. int.]: 138 (M+) [8], 120 (M-H2O+) [5], 107 (M-CH3O+) [100], 77 (C6H5+) [48]; IR vmax (CHCl3) cm-1: 3350 (OH), 3100, 2980, 1493, 1454, 1212, 1150; retention time: 14.00 min.
3-phenyl propyl acetate (E): 1H NMR (CDCl3, 300 MHz): δ 1.99 (td, 2H, J = 7.4, 6.6), 2.07 (s, 3H), 2.72 (t, 2H, J = 7.4), 4.12 (t, 2H, J = 6.6), 7.20-7.60 (m, 5H); 13C NMR (CDCl3, 75 MHz): δ 21.3 (CH3), 30.7 (C2), 32.6 (C1), 64.2 (C3), 126.5 (C4'), 128.8 (C3', 5'), 128.9 (C2', 6'), 141.7 (C1'), 171.4 (C=O); EI-MS, m/z [rel. int.]: 118 [71], 117 [100], 91 (C7H7+) [57], 77 (C6H5+) [10], 65 [13], 43 [19]; IR vmax (CHCl3) cm-1 3076-3027 (=C-H), 2934, 2853, 1728 (C=O), 1602 (C=C), 1494, 1263 (C-O), 1031, 737, 697; retention time: 16.92 min.
Similarly, (F) was identified by comparison of mass spectra with that in the NIST 2002 computerized mass spectral library.
Hydrocinnamic acid (F): EI-MS, m/z [rel. int.]: 150 (M+) [50], 105 (M-HCO2+) [19], 104 [61], 103 [16], 91 (C7H7+) [100], 78 [17], 77 (C6H5+) [20], 65 [12], 51 [12]. Retention time: 14.20 min.
Time-course experiment
Precultured C. acutatum was transferred into fourteen 500 mL Erlenmeyer flask containing 125 mL of Czapeck-Dox liquid medium and substrate (A), and stirred under the same conditions as for preculture. The organism was cultivated for 336 hours. The culture medium from each flask was removed daily. It was saturated with NaCl and extracted with CH2Cl2; and the solvent was subsequently evaporated. These extracts were analyzed by means of TLC, GC, and GC-MS. The ratios between the substrate and metabolic products were determined on the basis of GC peak areas. Control cultivation with no substrate was also performed.
Effect of culture medium on the biotransformation
This study was performed to determine the more suitable medium to achieve the transformation of cinnamyl alcohol to 2-PE by C. acutatum. Biotransformations using Sabouraud (peptone from casein, 10 g/L; anhydrous alpha-D(+)-glucose, 40g/L) and PDB (potato, 200 g/L; anhydrous alpha-D(+)-glucose, 20 g/L) liquid media were performd. Portions of 1 mL of the mycelia were transferred to inoculate 500 mL flasks, each containing 125 mL of each medium and substrate (A). Cultivation was carried out at room temperature and stirring (reciprocating shaker, 120 rpm) for 336 h. The culture medium from each f lask was removed daily under the same conditions as for the Time Course Experiment. Then, the culture medium was saturated with NaCl and extracted with CH2Cl2 (3x100 mL). Organic extracts were dried over anhydrous Na2SO4 and concentrated in vacuo. The progress of the biotransformation was monitored through GC–MS. Culture controls, in which the microorganism was grown under identical conditions (without substrate), were carried out.
RESULTS AND DISCUSSION
Antifungal bioassay
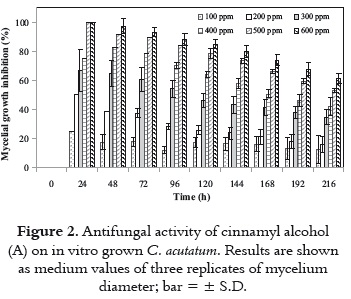
To determine the concentration to use in the biotransformation process, the antifungal activity of cinnamyl alcohol against C. acutatum was examined. Cinnamyl alcohol (A) possesses a moderate activity against the plant pathogenic fungus as shown in figure 2. The fungus C. acutatum was significantly inhibited at concentrations of 300 ppm and above. The growth inhibition percentage after 120 h was only 18% at 100 ppm, but it reached 78% and 85% at 500 and 600 ppm, respectively.
Cinnamyl alcohol presented a total growth inhibition of C. acutatum during the first 48 or 72 hours at 600 ppm. However, the inhibitory effect of (A) diminished with time, an aspect which suggests that the fungus has a detoxification mechanism. Based on figure 2, a concentration of cinnamyl alcohol of 400 ppm to perform the biotransformation was selected. This concentration of (A) was able to inhibit nearly 60% of fungal growth after 120 h. Therefore, C. acutatum was incubated with the substrate at 400 ppm for 336 h on a shaken culture (see Materials and Methods).
Biotransformation: identified and isolated compounds
To isolate the metabolic products, a preparative incubation of (A) in Czapeck-Dox liquid medium using C. acutatum was performed. The plant pathogenic fungus was incubated with cinnamyl alcohol at 400 ppm for 336 h. The culture was filtered. The filtrate was then extracted with CH2Cl2 and purified by means of column chromatography. A comparison through TLC and GC between the extract obtained from the biotransformation, and the control showed that C. acutatum transformed (A) into various metabolites. Three metabolic products (B), (C) and 2-PE were isolated and their structures were elucidated on the basis of spectral data, corresponding to 3-phenyl-1-propanol, 1-phenyl- 1,3-propanediol, and 2-phenylethanol, respectively. A small amount of biotransformation products (D), (E) and (F) was detected by means of TLC and GC analysis. Metabolic compounds were not detected through TLC or GC analyses of the culture of C. acutatum to which no substrate (A) was added. In addition, many minor metabolites were detected.
Interestingly, the phytopathogenic microorganism was able to reduce the double bond to give the major product 3-phenyl-1-propanol (B). This compound is used as a cosmetic and perfume ingredient (21). Additionally, the metabolite (C) was formed by hydration from cinnamyl alcohol. This diol has been reported as an intermediate in the synthesis of some important pharmaceuticals for the treatment of psychiatric disorders (depression, anxiety, alcoholism) and metabolic problems (obesity and bulimia), such as norfluoxetine and fluoxetine (22). Metabolic compounds (B) and (C) were previously obtained by microbial transformation of cinnamaldehyde using C. acutatum as a biocatalyst (20). Moreover, it had been reported that metabolites 2-PE and (D) were formed on the microbial transformation of acetophenone by C. acutatum (18) and Botryodiplodia theobromae (23).
Time-course experiment
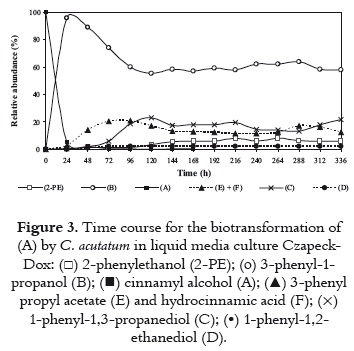
To evaluate the time course of relative abundance between substrate and metabolic products, (A) was incubated with the microorganism for 336 h. Every day, the culture medium from one flask was removed and extracted with CH2Cl2. Then, all extracts were analyzed through TLC and GC. Substrate (A), and metabolic products (B), (C), 2-PE, (D), (E), and (F) were quantitatively measured through GC. As it is shown in figure 3, the substrate (A) was mainly transformed into (B), and about 95% of cinnamyl alcohol was consumed in 24 h. Under the conditions used, the alcohol (B) reached about 90% of the products in 24 h and then decreased rapidly until the fifth day; whereas metabolic compounds (E) and (F) increased quickly after 24 h, obtaining the higher concentration on the fourth day. In addition, the diol (C) appeared after 48 h, and increased to about 23% after 120 h. The increase coincides with the decline of the relative abundance of (B). The metabolite 2-PE was produced after 120 h, and its relative abundance increased softly to reach almost 8% at day 9. The relative abundance of metabolite (D) was always lower than 5%.
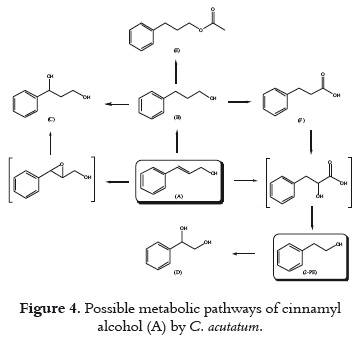
The metabolic pathways for the biotransformation of (A) by C. acutatum were proposed according to the time course experiment and the structures of the metabolites identified. The substrate was hydrogenated at the double bond by an enoate reductase (24) to give the major metabolic product (B) as described in figure 4. The diol (C) was considered to be produced through two approaches [I, (A)  (C); II, (A)
(C); II, (A) (B)
(B) (C)]. In route I, (C) was possibly formed via epoxidation by monooxygenases and subsequent hydrogenation of the epoxide. Although the intermediate epoxide could not be detected, it was previously reported for the biotransformation of cinnamaldehyde by C. acutatum (20). Alternatively, cinnamyl alcohol could be reduced to give (B), and subsequently hydroxilated in the benzilic position to produce the diol. This approach is based on the fact that (C) kept increasing although substrate (A) had disappeared. Probably both pathways were present. Metabolites (E) and (F) were formed from (B) through esterification (acetylation) with lipases, and oxidation by oxidoreductases (alcohol and aldehyde dehydrogenase), respectively. Even though (E) and (F) can proceed by acetylation and oxidation of (A), and subsequently hydrogenation of the double bond, neither cinnamyl acetate nor cinnamic acid was detected.
(C)]. In route I, (C) was possibly formed via epoxidation by monooxygenases and subsequent hydrogenation of the epoxide. Although the intermediate epoxide could not be detected, it was previously reported for the biotransformation of cinnamaldehyde by C. acutatum (20). Alternatively, cinnamyl alcohol could be reduced to give (B), and subsequently hydroxilated in the benzilic position to produce the diol. This approach is based on the fact that (C) kept increasing although substrate (A) had disappeared. Probably both pathways were present. Metabolites (E) and (F) were formed from (B) through esterification (acetylation) with lipases, and oxidation by oxidoreductases (alcohol and aldehyde dehydrogenase), respectively. Even though (E) and (F) can proceed by acetylation and oxidation of (A), and subsequently hydrogenation of the double bond, neither cinnamyl acetate nor cinnamic acid was detected.
The metabolic product 2-PE was considered to be possibly derived from (B) via oxidation [(B) (F)], followed by hydroxylation of the alpha carbon to carbonyl group, and finally decarboxylation. However, the intermediate 3-phenyllactic acid was not detected. Also, another possible pathway leading to 2-PE synthesis is from cinnamyl alcohol via phenylpyruvate, decarboxylation to phenylacetaldehyde by a decarboxylase, and subsequent reduction to 2-PE by a dehydrogenase. A similar pathway beginning from L-Phenylalanine (L-phe) has been proposed for microorganisms, especially yeasts, which are capable of producing 2-PE by normal metabolism (a biochemical pathway) (7, 25). This commonly accepted route for yeasts from L-phe to 2-PE was called the Ehrlich pathway. Nevertheless, in this study neither phenylpyruvate nor phenylacetaldehyde was detected. Meanwhile, the glycol (D) was apparently formed from 2-PE; this transformation occurs in a very low proportion which had been previously reported (18). Due to the commercial importance of 2-PE, a study of the culture medium effect on biotransformation of cinnamyl alcohol was carried out.
(F)], followed by hydroxylation of the alpha carbon to carbonyl group, and finally decarboxylation. However, the intermediate 3-phenyllactic acid was not detected. Also, another possible pathway leading to 2-PE synthesis is from cinnamyl alcohol via phenylpyruvate, decarboxylation to phenylacetaldehyde by a decarboxylase, and subsequent reduction to 2-PE by a dehydrogenase. A similar pathway beginning from L-Phenylalanine (L-phe) has been proposed for microorganisms, especially yeasts, which are capable of producing 2-PE by normal metabolism (a biochemical pathway) (7, 25). This commonly accepted route for yeasts from L-phe to 2-PE was called the Ehrlich pathway. Nevertheless, in this study neither phenylpyruvate nor phenylacetaldehyde was detected. Meanwhile, the glycol (D) was apparently formed from 2-PE; this transformation occurs in a very low proportion which had been previously reported (18). Due to the commercial importance of 2-PE, a study of the culture medium effect on biotransformation of cinnamyl alcohol was carried out.
Influence of the culture medium
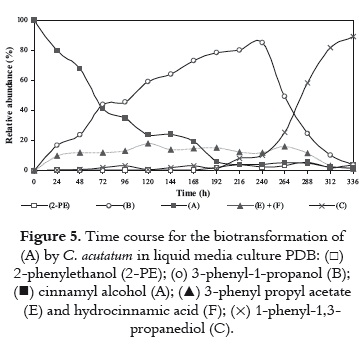
The effect of the culture medium on the biotransformation of cinnamyl alcohol by C. acutatum was investigated. Microbial transformations using PDB, and Sabouraud media were performed. According to figure 5, biotransformation on PDB medium showed to be slower than on Czapeck-Dox medium; substrate (A) was slowly converted by C. acutatum (~80% after 168 h). Compound (B), was the major metabolic product during the first 264 h, increasing to about 85% in 240 h. Then, (B) decreased rapidly, whereas (C) increased reaching nearly 90% of relative abundance. It seems remarkable that in PDB medium, the diol (C) reaches levels as different (~90%) in comparison with the Czapeck-Dox medium (<20%). Also, metabolite 2-PE was produced in small amounts (<6%) during all processes, and (D) was not detected.
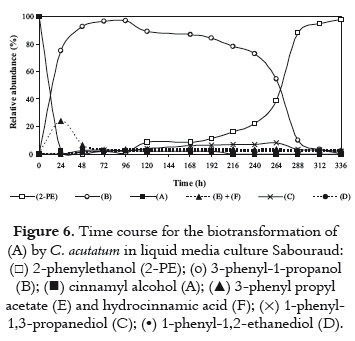
Otherwise, Sabouraud liquid medium demonstrated to be more suitable for transformation of (A) to 2-PE than Czapeck-Dox medium as shown in figure 6. In this culture medium, the substrate was almost completely biotransformed by C. acutatum within 24 h. Metabolite (B) was the main metabolic product during the first 264 h; it presented a relative abundance of between 70% and 90% during 24 and 240 h, reaching the maximum concentration at 96 h. Then (B) decreased, indicating the formation of other metabolites; whereas 2-PE increased rapidly, and was produced as a major metabolite after 264 h, accounting for about 90% of the metabolic products. Compounds (E) and (F) appeared within 24 h, reaching together a maximum relative abundance of 24%. After that, (E) and (F) declined to about 5% between 72 and 336 h. It seems noteworthy that some minor metabolites previously detected on Czapeck-Dox medium were not detected on Sabouraud medium.
Such specificity of the medium for transformation has also been reported by Gurran (26). The inconsistency in the production of metabolites in different media may be due to the suitability of that medium to induce enzymes of C. acutatum for the production of metabolites. Although the influence of the culture medium on the production of 2-PE was not so clear, the results obtained with Sabouraud were better than those obtained with Czapeck-Dox and PDB. Biotransformation using Sabouraud medium after 264 h of incubation can provide a simple and efficient way to obtain 2-PE with high yield. However, further investigations are needed to produce 2-PE in large quantities by optimizing the conditions of the biotransformation.
CONCLUSIONS
This paper demonstrates the ability of C. acutatum to transform cinnamyl alcohol. From the microbial transformation, six compounds were isolated and identified: 3-phenyl-1-propanol, 1-phenyl-1,3-propanediol, 2-phenylethanol, 1-phenyl-1,2-ethanediol, 3-phenyl propyl acetate, and hydrocinnamic acid. Thus, C. acutatum was able to reduce the double bond, and oxidize the side chain. Two possible metabolic pathways for 2-phenylethanol synthesis from cinnamyl alcohol are either through 3-phenyllactic acid or phenylpyruvate, followed by decarboxilation to afford phenyl acetaldehyde and reduction to 2-PE. Due to the commercial importance of 2-phenylethanol, a time course experiment using different culture media was carried out. Results indicate that after 288 hours of biotransformation using Sabouraud medium, the conditions are suitable for maximum transformation of cinnamyl alcohol to 2-phenylethanol by C. acutatum.
ACKNOWLEDGMENT
Special thanks to DIME (Dirección de Investigación Sede Medellín) and Universidad Nacional de Colombia for their financial support.
REFERENCES
1. Mookherjee BD, Wilson RA. Benzyl alcohol and β-phenethyl alcohol. Kirk-Othmer encyclopedia of chemical technology. 1st ed. Vol. 2. New York: John Wiley & Sons; 2000. p. 483–489. [ Links ]
2. Mei J, Min H, Lü Z. Enhanced biotransformation of L-phenylalanine to 2-phenylethanol using an in situ product adsorption technique. Process Biochem. 2009 Aug; 44 (8): 886–890. [ Links ]
3. Rode CV, Kshirsagar VS, Rane VH, Chaudhari RV., inventors; Council of Scientific and Industrial Resesearch, assignee. Process for preparation of 2-phenyl ethanol. US Patent 6,166,269. 2000 Dec 26. [ Links ]
4. Savina JP, Kohler D, Brunerie P, inventors; Pernod Ricard, assignee. Method for extracting 2-phenylethanol. US Patent 5,965,780. 1999 Oct 12. [ Links ]
5. Sendovski M, Nir N, Fishman A. Bioproduction of 2-phenylethanol in a biphasic ionic liquid aqueous system. J Agr Food Chem. 2010 Feb; 58 (4): 2260–2265. [ Links ]
6. Bauer K, Garbe D, Surburg H. Common fragrance and flavor materials. Preparation, properties and uses. Weinheim: Wiley-VCH; 2001. 304p. [ Links ]
7. Etschmann MMW, Bluemke W, Sell D, Schrader J. Biotechnological production of 2-phenylethanol. Appl Microbiol Biot. 2002 Jun; 59 (1): 1–8. [ Links ]
8. Etschmann MMW, Sell D, Schrader J. Medium optimization for the production of the aroma compound 2-phenylethanol using a genetic algorithm. J Mol Catal B-Enzym. 2004 Jun; 29 (1-6): 187–193. [ Links ]
9. Janssens L, De Pooter HL, Schamp NM, Vandamme EJ. Production of flavours by microorganisms. Process Biochem. 1992 Jul; 27 (4): 195-215. [ Links ]
10. Kawabe T, Morita H. Volatile components in culture fluid of Polyporus tuberaster. J Agr Food Chem. 1993 Nov; 41 (4): 637-640. [ Links ]
11. Fabre C, Blanc P, Goma G. Production of benzaldehyde by several strains of Ischnoderma benzoinum. Sci Aliment. 1996 Jan; 16 (1): 61-68. [ Links ]
12. Lomascolo A, Lesage-Meessen L, Haon M, Navarro D, Antona C, Faulds C, et al. Evaluation of the potential of Aspergillus niger species for the bioconversion of L-phenylalanine into 2-phenylethanol. World J Microb Biot. 2001 Feb; 17 (1): 99–102. [ Links ]
13. Fabre C, Blanc PJ, Goma G. Production of 2-phenylethyl alcohol by Kluyveromyces marxianus. Biotechnol Progr. 1998 Mar-Apr; 14 (2): 270-274. [ Links ]
14. Peres NA, Timmer LW, Adaskaveg JE, Correll JC. Lifestyles of Colletotrichum acutatum. Plant Dis. 2005 Aug; 89 (8): 784-796. [ Links ]
15. García-Pajón CM, Hernández-Galán R, Collado IG. Biotransformations by Colletotrichum species. Tetrahedron-Asymmetr. 2003 May; 14 (10): 1229-1239. [ Links ]
16. Krishna-Kumari GN, Masilamani S, Ganesh MR, Aravind S. Microbial transformation of zaluzanin-D. Phytochemistry. 2003 Apr; 62 (7): 1101-1104. [ Links ]
17. Wilson MR, Gallimore WA, Reese PB. Steroid transformations with Fusarium oxysporum var. cubense and Colletotrichum musae. Steroids. 1999 Dec; 64 (12): 834-843. [ Links ]
18. Aristizábal DA, Lezcano CS, García CM, Durango DL. Biotransformación de los sustratos 2-feniletanol y acetofenona con el hongo fitopatógeno Colletotrichum acutatum. Rev Colomb Quím. 2008 Jun-Abr; 37 (1): 7-19. [ Links ]
19. Afanador-Kafuri L, Minz D, Maymon M, Freeman S. Characterization of Colletotrichum isolates from Tamarillo, Passiflora, and Mango in Colombia and identification of a unique species from the genus. Phytopathology. 2003 May; 93 (5): 579-587. [ Links ]
20. Correa YM, Durango DL, García CM. Transformación microbiana del arilpropanoide cinamaldehído con el hongo fitopatógeno Colletotrichum acutatum. Vitae. 2009 Ene-Abr; 16 (1): 83–91. [ Links ]
21. Behan JM, Clements CF, Hooper DC, Martin JR, Melville JB, Perring KD., inventors; Lever Brothers Company, Division of Conopco, Inc., assignee. Perfume compositions. US Patent 5,554,588. 1996 Sep 10. [ Links ]
22. Pradeep K, Rajesh KU, Rajesh KP. Asymmetric dihydroxylation route to (R)-isoprenaline, (R)-norfluoxetine and (R)-fluoxetine. Tetrahedron: Asymmetr. 2004 Dec; 15 (24): 3955–3959. [ Links ]
23. Velasco R, Valverde ID, Durango DL, García CM. Biotransformación de los compuestos 2-feniletanol y acetofenona mediante el hongo fitopatógeno Botryodiplodia theobromae. Vitae. 2007 Jul-Dic; 14 (2): 43-50. [ Links ]
24. Hall M, Hauer B, Stuermer R, Kroutil W, Faber K. Asymmetric whole-cell bioreduction of an α,β-unsaturated aldehyde (citral): competing prim-alcohol dehydrogenase and C–C lyase activities. Tetrahedron: Asymmetr. 2006 Nov; 17 (21): 3058–3062. [ Links ]
25. Hazelwood LA, Daran JM, van Maris AJ, Pronk JT, Dickinson JR. The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl Environ Microb. 2008 Apr; 74 (8): 2259-2266. [ Links ]
26. Gurram SP, Kollu NR, Sivadevuni G, Solipuram MR. Biotransformation of albendazole by Cunninghamella blakesleeana: influence of incubation time, media, vitamins and solvents. Iran J Biotechnol. 2009 Oct; 7 (4): 205-215. [ Links ]
Received: 07 March 2009
Accepted: 09 August 2010