Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.17 no.3 Medellín Sept./Dec. 2010
REVIEWS
PRO-APOPTOTIC EFFECTS OF APPLE PROCYANIDINS IN HUMAN COLON CANCER CELLS AND THEIR DERIVED METASTATIC CELLS
EFECTO PRO-APOPTÓTICO DE PROCIANIDINAS DE MANZANA EN CÉLULAS HUMANAS DE CÁNCER DE COLON Y SUS CÉLULAS METASTÁSICAS DERIVADAS
María E. MALDONADO C.1, Francis RAUL2,3
1 Grupo de Alimentación y Nutrición Humana. Escuela de Nutrición y Dietética. Universidad de Antioquia. Medellín, Colombia. mariaele@quimbaya.udea.edu.co.
2 Université de Strasbourg Unit 4438, Faculté de Médecine, 67000 Strasbourg, France.
3 Institut de Recherche contre les Cancers de l'Appareil Digestif (IRCAD), 67000 Strasbourg, France.
ABSTRACT
Apples are a rich source of Procyanidins (Pcy) which are able to inhibit colon carcinogenesis in animal models, but the mechanisms through which this occurs are not well understood.The evidence obtained in our laboratory and by other researchers, which shows that Pcy trigger apoptosis through different mechanisms in human colon adenocarcinoma SW480 cells and their derived-metastatic SW620 cells is reviewed in this paper. In the apoptosis induced by Pcy, the polyamine metabolism is involved, but it is not present in SW480 cells. There is a differential sensitivity of both cells lines to the activation of TRAIL-death receptors. Pcy enhance the sensitivity of SW480 cells to TRAIL by activating the extrinsic apoptotic pathway, and overcome TRAIL-resistance in SW620 cells involving a cross-talk between the extrinsic and intrinsic pathways; and a Pcy-induced ROS production favoring mitochondria disruption. In addition, Pcy activate Fas receptor in SW480 cells, whereas SW620 cells are Fas-resistant despite the up-regulated Fas expression. Surprisingly, activation of the Fas receptor-mediated apoptosis by Pcy is observed in SW620 cells after inactivation of TRAIL-death receptors, suggesting that Fas-resistant phenotype may be associated with alterations in downstream events between TRAIL-death and Fas receptors. These data highlight the potential interest of apple Pcy in colon cancer prevention and therapy (combination therapy).
Key words: apoptosis, colon cancer, flavonoids, TRAIL, Fas.
RESUMEN
Las manzanas son fuente rica en Procianidinas (Pcy), inhiben la carcinogénesis del colon en modelos animales aunque los mecanismos no son bien comprendidos. Esta revisión presenta evidencia sobre los efectos pro-apoptóticos de Pcy por diferentes mecanismos en células humanas de adenocarcinoma de colon SW480 y sus derivadas metastáticas SW620. En la apoptosis inducida por Pcy en células SW620 participa el metabolismo de poliaminas, pero no en células SW480. Existe sensibilidad diferencial de ambas líneas a la activación de los receptores de muerte-TRAIL. Pcy aumenta la sensibilidad de SW480 a TRAIL activando la vía extrínseca, y sobrepasa la resistencia a TRAIL en células SW620 mediante interacción entre las vías extrínseca e intrínseca, y producción de especies reactivas del oxígeno (ROS) con daño mitocondrial. Pcy activan el receptor Fas en células SW480, mientras que las células SW620 son Fas-resistentes a pesar del aumento en la expresión de Fas. La activación de la apoptosis vía Fas por Pcy se observa en células SW620 después de inactivar los receptores de muerte-TRAIL, sugiriendo que el fenotipo Fas-resistente podría estar asociado con alteraciones corriente-abajo entre los receptores TRAIL y Fas. Estos datos resaltan el potencial interés en las Pcy de manzana para la prevención y terapia combinada del cáncer de colon.
Palabras clave: apoptosis, cáncer de colon, flavonoides, TRAIL, Fas.
INTRODUCTION
Epidemiological studies support an inverse relationship between regular consumption of fruits, vegetables, and the risk of CRC (Colorectal Cancer). Individual non-nutritive polyphenolic compounds present in fruits, identified as inhibitory agents of colon carcinogenesis, can be divided into various classes on the basis of their molecular structure, with flavonoids being one of the main groups occurring in human diet. Apples are a rich source of flavonoids, and its major subclass is flavanols, which contain monomers (epicatechin and catechin) and polymeric forms (procyanidins). An in vitro model of colon carcinogenesis that represents the progression from a primary tumor (SW480 cells) to metastatic disease (SW620 cells) was employed, and it was found that apple procyanidins can directly or indirectly influence some important targets involved in apoptosis, such as polyamines metabolism, TRAIL-death receptor pathway, Fas-receptor pathway, and mitochondrial integrity. Evidences presented in this review encourage knowing that a dietary agent such as apple Pcy presents multipotent anti-cancer properties and may represent a promising agent for the chemoprevention of colon cancer through the induction of apoptosis.
General characteristics of procyanidins
Procyanidins (Pcy), also known as condensed tannins, represent the second most abundant class of plant polyphenols (1). Pcy are widely distributed in the peel of fruits, legumes, cereal grains, and a variety of beverages including wine, beer, tea, cocoa, and cider. Their content is decreased by industrial food processing methods as fruit peeling, decortications, juice filtration, maceration, drying or long-term storaging. For example, natural cloudy apple juice contains about 2.5-fold more Pcy than processed clear apple juice (2, 3). They are of interest in nutrition and medicine due to their potent antioxidant capacity and possible effects on human health as they reduce the risk of chronic diseases such as cardiovascular diseases and cancer.
Pcy are di-, tri- and oligomeric condensation products of flavan-3-ol monomers of (+)-catechin and (-)-epicatechins; they generally possess 12–16 phenolic OH-groups and 5-7 aromatic rings/1000 units of relative molecular mass, and they also possess an average molecular weight of 1000-6000. Their mean degree of polymerization (DP) in foods has rarely been determined, however, in cider apples the DP ranges from 4 to 11 (4).
The Pcy content of red wine, chocolate, cranberry juice and cloudy apple juice (5) and several varieties of apples (6) has been determined in an analytical study. On average, apples contain a large Pcy content per serving (147.1 mg), this corresponds to 63-77% of apple polyphenols. Moreover, a serving size of cloudy apple juice contains 48-61% of Pcy. The Pcy content varied greatly between apple samples (12.3–252.4 mg/serving) with the highest amounts on average observed for the Red Delicious (207.7 mg/ serving) and Granny Smith (183.3 mg/serving) varieties; and the lowest amounts in the Golden Delicious (92.5 mg/serving) and McIntosh (105.0 mg/serving) varieties (5, 6).
Apoptosis
Apoptosis is a physiological process in which cells are removed after exposing them to toxic compounds; it also takes place during development and in degenerative disorders. Apoptosis is a nontoxic model of cell death which affects single cells in the midst of living tissues without eliciting an inflammatory response (7). Apoptosis is considered to be one of the important targets in a cancer preventive approach, which provides a physiological mechanism for the elimination of abnormal cells. In studies of animal colon cancer, the experimental enhancement of crypt-cell apoptosis has shown to suppress the induction of neoplasia by chemical carcinogens (8, 9).
Apoptosis is characterized, at the morphological level, by cell shrinking, membrane blebbing, nuclear pyknosis, chromatin condensation, and cellular fragmentation into so-called apoptotic bodies, rapidly phagocyted and digested by macrophages (10). These changes are preceded by biochemical events such as the redistribution of membrane lipids, the loss of mitochondrial membrane potential, and the fragmentation of DNA at internucleosomal sites (11). There are two main pathways involved in the induction of apoptosis; a death receptor-pathway (the extrinsic pathway) and a mitochondrial pathway (the intrinsic pathway). The apoptotic signal involves the activation of many proteins that are part of two major families: the caspases and the Bcl-2 proteins. Caspases play an important role in the degradation of cellular organelles, whereas Bcl-2 proteins participate in the maintenance and propagation of the signal (7).
The polyamines metabolism and apoptosis triggered by apple Pcy
The putrescine (Put), spermidine (Spd) and spermine (spm) are aliphatic amines known as polyamines, which are formed and stored by nearly all eukaryotic cells. They are required for optimal growth in cells. The loss of these polyamines results in the inhibition of cell proliferation and differentiation, and sometimes even cell death (necrosis and/or apoptosis). It has been shown that when quiescent cells are stimulated to growth, the biosynthesis of polyamines increases before the synthesis of DNA, RNA and proteins for cell cycle (12, 13). At physiological pH, the polyamines are nearly completely protonated which enables them to form ion bonds with a wide variety of anionic-binding, such as nucleic acids and proteins, stabilizing their conformations (14).
An important function of the polyamines is gene regulation. The depletion of polyamines affects the expression of numerous genes, as well as of protein factors involved in the cell cycle growth and regulation, and in programmed cell death. Investigations of the role of the polyamines as modulators of the binding of transcription factors and related proteins to relevant DNA sites are therefore of great interest. Several DNA–protein interactions are modulated by polyamines, for example, spermine improves the binding of protein to DNA. Another function of polyamines is their role as precursors of biologically active compounds such as Hypusine (N-ε-(4-amino-2- hydroxybutyl-lysine), the prosthetic group of the eukaryotic initiation factor eIF-5A, which appears to be involved in the control of cell proliferation and apoptosis. Since spermidine is a precursor of hypusine, the depletion of the spermidine pools prevents the formation of active eIF5A, therefore, cells stop growing (12-14).
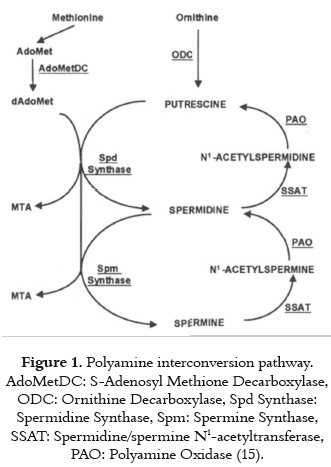
The polyamines metabolism consist of biosynthesis and catabolic or retroconversion pathways shown on figure 1. The two key enzymes involved in polyamine biosynthesis are ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (AdoMetDC). ODC catalyzes the formation of putrescine from L-ornithine, and AdoMetDC decarboxylates S-adenosylmethionine (AdoMet). The product of this reaction, decarboxylated S-adenosylmethionine (dcAdoMet), is the aminopropyl group donor for spermidine and spermine synthesis. In the catabolic pathway (or retroconversion pathway) acetylated polyamines are formed by spermine/spermidine acetyltransferase (SSAT) and are used as substrates by a flavin-dependent polyamine oxidase (PAO), which catalyzes their conversion back to spermidine, and finally to putrescine (14).
The polyamine metabolism differs in various aspects in SW480 and SW620 cells (16, 17). The polyamine metabolism has been studied as a potential target of apple Pcy because they are involved in cell proliferation and in the maintenance of cell viability. Apple Pcy-induced apoptosis in SW480 and SW620 cells is potentiated by the use of MDL 72527 (MDL), a specific inhibitor of PAO, in the metastatic SW620 cells (18) but not in SW480 cells. This difference in sensitivity to MDL in SW480 and SW620 cells is probably not a function of PAO activity, which presents similar activity in both cell lines (16). Therefore other aspects may be involved, such as the intracellular pool of polyamines and their acetylated forms.
Pcy-induced apoptosis in SW620 cells is accompanied by a decreased intracellular pool of polyamines and a higher accumulation of acetylated polyamines, respectively indicating a reduction of polyamine biosynthesis and an enhanced polyamine catabolism (18). This is correlated to a previous report by Gossé et al., 2005 (8), showing Pcy down-regulated ODC and AdoMet DC activities in SW620 cells, the two enzymes of polyamine biosynthesis. These effects of Pcy on polyamines metabolism are enhanced in presence of MDL 72527, a specific inhibitor of PAO activity. The depletion of the intracellular pool of polyamines in presence of Pcy/MDL led to apoptosis, and the addition of exogenous polyamines to SW620 cell culture inhibit the potentiation by MDL of Pcytriggered apoptosis, which confirms the importance of polyamines (putrescine, spermidine and spermine) for cell proliferation and maintenance of cell viability (18).
The massive formation of acetylated polyamines may contribute to cell death due to the depletion of Acetyl-CoA (19). In addition, there is a discussion speculating the possibility that the accumulation of intracellular N-acetylated polyamines affects histone acetylation by competing with the acetyl-CoA: spermidine N8-acetyltransferase, an enzyme that also has histone acetylating properties (20). However, findings in SW620 cells suggest that in the presence of Pcy, MDL might favor the hyperacetylation of the promoters of TRAIL-death receptors, leading to the up-regulation of DR4/DR5 expression (21, 22); which may be controlled at the transcriptional level, favored by an inhibition of 50% nuclear Histone deacetylase (HDAC) activity caused by Pcy/MDL. Under these conditions, the activation of the extrinsic apoptotic pathway has been induced through the TRAIL-death receptors, confirmed by the reduction of Pcy/MDL-induced cell death after a specific inhibition of DR4/DR5 receptors (18). The SW620 cell line is normally resistant to TRAIL-death receptor mediated apoptosis because they do not express DR4 and DR5 receptors at the cell surface (23). Thus, the activation by Pcy/MDL of TRAIL-death receptor mediated apoptosis through the modulation of polyamine metabolism can be of great interest in chemoprevention, since this pathway can preferentially activate apoptosis in cancer cells but not in normal non-cancerous cells (24). Furthermore, the Pcy/MDL combination could potentiate the effect of TRAIL exogenous and/or the TRAIL produced by cells of innate immune system (25).
The apoptotic pathway activated by Pcy only in SW620 cells is different to the one observed in presence of MDL (18). Pcy activate the intrinsic mitochondrial apoptotic pathway through the alteration of mitochondrial membrane potential, involving the activation of caspases-9 and -3. These effects caused by the Pcy-enhanced ROS production (26) are prevented by MDL. Indeed, MDL inhibits ROS generated through the activation of polyamine catabolism caused by Pcy. This increase of intracellular ROS may be a way to kill cancer cells by activating the mitochondrial permeability transition (27, 28).
Apple Pcy also induce apoptosis in SW480 cells, although this effect is not enhanced by MDL 72527 as observed in SW620 cells, which suggest that Pcyinduced apoptosis in SW480 cells is unrelated to the production of H2O2 by the oxidation of N1-acetyl derivatives of spermidine and spermine. This reaction is catalyzed by the PAO when these acetylated polyamines are excessively formed by the SSAT enzyme (15). SW480 cells seem to be less sensitive to combined treatment of Pcy and MDL in comparison to SW620 cells, despite the similar PAO activity in both cell lines under basal conditions (16). This difference on the apoptotic effects observed with Pcy/MDL in SW480 cells may be related to low polyamine biosynthetic activity and low intracellular content of acetylated forms of the polyamines observed in these cells (25). Indeed, if Pcy down-regulate polyamine biosynthesis (8, 15), which already is significantly low under basal conditions in SW480 cells (16, 17); the levels of non-acetylated forms of polyamines would probably be lower in these cells.
In SW480 cells, Pcy only or combined with MDL activate an extrinsic apoptotic pathway by up-regulating expression of TRAIL-death receptors DR4/DR5. We investigated whether the activation of TRAIL-death receptor pathway under these conditions was associated to a reduced activity of HDAC. Pcy, as well as MDL, used individually do not affect the enzyme activity; whereas MDL in combination with Pcy reduced by 20% HDAC activity, which could favor hyperacetylation of the promoters of DR4/DR5 receptors. However, no significant difference is observed in the percentage of SW480 cells expressing DR4/DR5 receptors after Pcy only or combined Pcy/MDL treatments, which suggests that the enhanced expression of TRAIL-death receptors at the cell surface of SW480 cells might be regulated at a post-transcriptional level (29). The alteration of the polyamine metabolism is a factor involved in the Pcy-induced apoptosis of SW620 metastatic cells, but not of SW480 colon cancer cells.
Activation of TRAIL-death receptor mediated pathway
Tumor necrosis factor a-related apoptosis-inducing ligand (TRAIL) is a death ligand expressed in the majority of human tissues. It has been demonstrated that four Death Receptors (DRs) are specifically bound to TRAIL: two cell death-inducing receptors (TRAIL-R1/DR4 and TRAIL-R2/DR5) and two non-cell-death-inducing receptors (TRAIL-R3/DcR1 and TRAIL-R4/DcR2), (27, 28). Like TRAIL, these receptors are expressed in a wide variety of tissues. However, it has been reported that TRAIL demonstrates selective toxicity to cancer cells, but not to normal cells (30, 31). DR4 and DR5 signal apoptosis through the interaction with FADD and caspase 8 (30, 32) may activate the intrinsic mitochondrial cell death pathway (33). In addition to apoptosis, TRAIL ligation of DR4 and DR5 can activate NF-kB and p53 (34, 35).
Once it was showed that Pcy can induce apoptosis in SW480 and SW620 cells through the activation of TRAIL-death receptors DR4/DR5, the mechanism through which DR4/DR5 could be activated downstream by Pcy only or in the presence of exogenous TRAIL was described in both cell lines. Pcy enhance the sensitivity to the apoptotic effects of TRAIL in SW480 cells and overcome TRAIL-resistance in SW620 cells (26). The co-administration of Pcy and TRAIL enhance apoptotic signaling, leading to nuclear DNA fragmentation in both cell lines compared to TRAIL alone (29).
In SW480 cells, the increased expression of DR4/DR5 receptors by Pcy at the cell surface enhanced the sensitivity to TRAIL. In these cells, the main apoptotic pathway activated by Pcy only and combined with TRAIL is the extrinsic pathway, involving the activation of caspase-8 and caspase-3. Mitochondrial dysfunctions observed in SW480 cells exposed to Pcy and Pcy/TRAIL is limited and do not cause the release of cytochrome c into cytosol and caspase-9 activation, because neither Bid protein nor Bcl-2/Bax are modified. In contrast, in the metastatic SW620 cells, Pcy initiate apoptosis through a crosstalk between the TRAIL-death receptor pathway and the intrinsic (mitochondrial) apoptotic pathway through an activation of the caspase-8, a reduction of full length Bid protein, and parallel to a progressive increase Bax protein and mitochondria membrane permeabilization. Such mitochondrial dysfunction favors the release of cytochrome c into cytosol, leading to the activation of caspase-9 and, consequently, caspase-3. In SW620 cells, these events are associated to ROS production induced by Pcy which are also enhanced in the presence of exogenous TRAIL (26).
The importance to activate the TRAIL apoptotic pathway for the treatment of cancer is highlighted by the recent introduction of TRAIL-receptor agonistic antibodies in human phase 1 trials (36, 37). However, most solid tumor cells are relatively resistant to TRAIL-induced apoptosis. Even though, numerous studies have shown that TRAIL resistance can be overcome by the combined application of chemotherapeutic drugs. Thus, finding natural phytoconstituents able to enhance the apoptotic effect of TRAIL, which is produced by cells of the immune system as macrophages or natural killer cells (25), and sensitizing TRAIL-resistant cancer cells represents an important potential strategy for cancer therapy.
Pcy, similarly to phytochemicals such as curcumin (38), quercetin (39, 40), apigenin (41), and resveratrol (42) augments TRAIL-mediated apop tosis in cancer cells by increasing the expression of death-receptors on the cell surface, improvement the transport of DR4/DR5 proteins into the cell surface. The failure in trafficking mechanisms for delivering TRAIL-death receptors may contribute to TRAIL-resistance in colon cancer cells (43). In SW480 cells, although the total amount of DR4 and DR5 transcripts is similar in control and treated cells, Pcy increase DR4 and DR5 expression at cell surface and sensitize SW480 cells to TRAIL. This effect was similar to the one observed by Jin et al., 2004 (44) using the glycosilation inhibitor tunicamicyn in SW480 TRAIL-resistant clones. In contrast to TRAIL-resistant SW620 cells, Pcy increased the levels of DR4/DR5 transcripts. This event is correlated to the enhanced localization of death receptors at the cell surface, which suggests that Pcy regulate post-transcriptional mechanisms involved in the delivery of death receptors to the cell membrane in SW480 cells (45). On the other hand, Pcy overcame TRAIL-resistance in SW620 cells by regulating the transcription and the intracellular transport mechanisms, leading to increase cell surface expression of TRAIL-death receptors.
It has been proposed that the basic expression level on the cell surface is not enough to determine TRAIL-sensitivity, but also the redistribution of TRAIL-death receptors to the cell membrane and the formation of lipid rafts, plasma membrane microdomains enriched with cholesterol, glycosphingolipids and caveolar-associated proteins, such as caveolin. All of them are able to regulate the efficacy of death receptor signaling throough the redistribution in the cell membrane (42). In colon cancer cells, resveratrol (46) and quercetin (40) induced DR4/DR5 receptor redistribution in lipid rafts colocalized with caveolin-1, becoming sensitive to TRAIL, this lipid raft formation was prevented by the cholesterol sequestering agent nystatin. Caveolin-1 is an integral membrane protein involved in cellular signaling transduction. This protein has been described as a marker for raft-associated caveolae which interacts with lipids such as cholesterol, which are both structural components of caveolae (47).
Pcy are able to activate the TRAIL-death receptor pathway through a lipid-raft independent mechanism in both cell lines. This is confirmed with the analysis of lipid-raft fractions of Pcytreated SW480 (45) and SW620 cells, where the levels of DR4, DR5 and caveolin were similar to the basal conditions. Caveolin protein can interact directly and inhibits or sequester the inactive form of many signaling molecules via the scaffolding domain (47). Therefore, the direct activation of the TRAIL-death receptor pathway by Pcy independent of lipid raft-formation, could be considered an interesting strategy for sensitizing SW480 and SW620 cells to TRAIL-death receptor mediated apoptosis, and overcoming the resistance mechanisms that suppress the signaling activity of TRAIL-death receptors. It has been proposed that antitumor properties of flavanols and Pcy can be associated to the oligomeric chain length which directly influences the interactions of the lipid bilayer and the membrane proteins (48).
In response to the interaction of Pcy with the cell membrane, DR4 and DR5 are activated, the procaspase- 8 is recruited to the DISC complex via binding to FADD (49) which results in caspase-8 activation. This is supported by the ability of a selective inhibitor of caspase-8 to reduce the induced apoptosis by Pcy only, or Pcy combined with TRAIL in SW480 cells, compared to SW620 cells (26). Caspase-8 has two important substrates, procaspase-3 and Bid protein. Caspase-3 is the main downstream effector caspase that cleaves the majority of the cellular substrate in apoptotic cells (50). Bid is the link in the crosstalk between the extrinsic and the intrinsic apoptotic pathways. Bid also disrupts mitochondria and favors the release of pro-apoptotic factors such as cytochrome c (51). Caspase-8 cleaves Bid as efficiently as it cleaves pro-caspase-3 (52). However, although caspase-8 is activated in both cell lines after Pcy and combined with TRAIL, Bid is cleaved only in SW620-treated cells. As a consequence, a decreased mitochondrial membrane potential is observed in these cells, leading to the release of cytochrome c into cytosol and to caspase-9 activation; which indicates that the mitochondrial pathway is activated in Pcy-induced apoptosis through the action of TRAIL-death receptors.
The reason for an inefficient cleavage of Bid protein in Pcy-treated SW480 cells is not the lack of caspase-8 enzyme activity. It may be influenced by posttranslational modifications of Bid (52), mutations to Bid cleavage sites (53), or the presence of a negative regulator (54) in SW480 cells. It has been demonstrated that the phosphorylation of Bid prevents caspase-8 cleavage (55). In addition, truncated Bid form (tBid) has been reported to be ubiquitinated and targeted for degradation (56). It has also been shown that in the SW480 cells, the overexpression of Bcl-2 blocked TRAIL-death receptor mediated apoptosis by inhibiting Bax translocation into mitochondria and reduced cytochrome c release. Thus, strategies to overcome the observed Bcl-2-mediated resistance to the mitochondrial pathway have the potential to greatly increase treatment efficacy. One possible approach involves the use of small molecules inhibitor that binds to Bcl-2 and inhibits it function (54).
Many studies have shown that cancer preventive agents induced apoptosis through the generation of ROS. Apoptosis induced by Pcy only or combined with TRAIL in SW620 cells also involves generation of ROS, which can result from the increased polyamine catabolism, as well as from the mitochondrial permeabilization caused by tBid and Bax (26). ROS produced in cytosol may also activate a cell intrinsic pathway of apoptosis by perturbing the mitochondrial function which is inhibited by MDL (18). Taken together, these observations suggest that ROS generation is another mechanism by which Pcy may sensitize TRAIL-resistant SW620 cells as observed in cancer cells treated with resveratrol (46) and curcumin (38). The accumulation of ROS leads to mitochondrial functional changes, such as the increased permeability transition pore opening and loss of Dym, which are events associated with the release of cytochrome c and caspase activation (27).
Modulation of Fas- receptor mediated apoptosis
Fas-receptor (CD95) is an integral cell membrane protein and a member of the TNF receptor family. Pcy sensitized SW480 cells to Fas receptormediated apoptosis. This sensibilization was associated with an up-regulated expression at the post-transcriptional level of the Fas receptor without significant changes at the transcriptional level, which suggests that the sensitizing mechanism of Pcy to Fas implies a favored delivery of the Fas receptor to the cell membrane. On the contrary, SW620 cells showed a Fas-resistant phenotype as it was described above (57), despite the up-regulation of Fas transcripts correlated with a huge expression of the receptor at the cell surface.
Tumours have developed multiple mechanisms for evading the surveillance of the immune system. Most cancer cells are relatively resistant to Fas-mediated apoptosis (58). This protects tumor cells from FasL expressed as a cytotoxic mediator by T cells (59) or NK cells (60) infiltrated into the tumor. Colon cancer cells have acquired defensive strategies (Fas resistance) against this effect by either down-regulating Fas-receptor (61) or by acquiring Fas-receptor signaling defects (58). A mutated intracytoplasmic domain of Fas receptor has been reported, precluding the establishment of a functional DISC (62). Some cancer types express high levels of Fas-associated phosphatase-1 (FAP-1) (63) which interacts with the C-terminal region of Fas, leading to the inhibition of downstream events. The microinjection of a tripeptide, corresponding to the three amino acids of Fas receptor C-terminal region, prevented this interaction and restored the Fas sensitivity of the colon cancer cell line (64).
The activation of Fas-receptor by apple Pcy was confirmed in both cell lines through two strategies: i) usising an antagonist antibody to Fas-receptor, anti-Fas ZB4; ii) Pcy combination with an agonist antibody of Fas-receptor, the anti-Fas CH-11 which reproduces the activation by FasL. Under these conditions, an increase in the number of hypodiploid cells, a loss of mitochondrial membrane potential and DNA fragmentation caused by the activation of Fas receptor have been observed in SW480 cells. The potentiated pro-apoptotic effects of anti-Fas CH-11 were enhanced by Pcy which are abrogated by the anti-Fas ZB4. We also observed in these cells that Pcy combined with anti-Fas CH-11 activate a type II (mitochondrial)-apoptotic pathway, which is an effect also observed for anti-Fas CH-11 in other experimental models (65). Whereas, the use of multimeric forms of Fas ligand induces a physiologically relevant apoptotic signaling type I (without mitochondria) pathway through the activation of the Fas receptor (66). In any case, these evidences supports the concept that Pcy may help cancer cells to recover Fas sensitivity, contributing to the elimination of tumor cells by FasL or by agonists of Fas-receptor.
On the contrary, in SW620 cells the anti-Fas CH-11 do not enhance the pro-apoptotic effects observed with Pcy only. However, in SW620 cells it is not excluded that the Fas-receptor might be implicated in the Pcy-induced apoptosis after a blockade of TRAIL-DR4/-DR5 receptors that induce its activation by Pcy. This fact suggests that Pcy are able to initiate a cross-talk between DR4/DR5 and Fas in metastatic cells. These findings suggest molecular defects at the level of Fas signal transduction. Thus, understanding the basis of the resistance of metastatic-derived colon cancer cells to Fas-mediated apoptosis might provide new strategies and treatment types for targeting death receptors in cancer cells (29).
In this study, we propose that Pcy may restore Fas-sensitivity in metastatic cells by overcoming several aspects, contributing to Fas-resistance: i) the ratio between DR4/DR5 and Fas receptors in cell membrane may play an important role in determining Fas-sensitivity. The number of Fas receptor relative to DR4/DR5 might not be sufficient to induce Fas-mediated apoptosis, since both types of receptors interact similarly with the adaptor protein FADD (67, 68) after their activation by the combined treatments Pcy /TRAIL or Pcy / anti-Fas CH-11; ii) DR4/DR5 and Fas receptors have different C-terminal tails. The corresponding region for DR4 and DR5 positively regulates FADD binding, caspase activation and apoptosis, whereas the Cterminal tail of Fas receptor has the opposite effect and inhibits the binding of FADD to the receptor death domain (67). We may hypothesize that the C-terminal tail of DR4 and DR5 receptors located outside the death domain could present additional regulatory sites for the activation of Fas receptor in order to overcome an inactivation of DR4/DR5 receptors (67). However, currently there is no evidence showing such a direct interaction between Fas and DR4/DR5 receptors; iii) Cross linking of FasL and TRAIL to their respective receptors activates PKC which in turn triggers anti-apoptotic mechanisms for the mitochondrial pathway (69). Thus, one may speculate about the possibility that the simultaneous inhibition with blocking antibodies for DR4/DR5 and Fas affects PKC activity, which can also be inhibited by apple Pcy in SW620 cells as described by Gossé et al., 2005 (8). However, all these aspects deserve further investigations.
CONCLUSIONS
The different apoptotic signaling pathways triggered by apple Pcy, demonstrated in the course of this review, are summarized on figures 2 and 3; where a differential induction of apoptosis between SW480 and SW620 cells is illustrated. The Pcy were able to induce apoptosis in SW480 cells through the extrinsic apoptotic pathway involving TRAIL-DR4/-DR5 and Fas receptors activated by a post-transcription mechanism. On the contrary, Pcy can induce apoptosis in SW620 cells through the activation of the TRAIL-DR4/-DR5 or the Fas apoptotic and intrinsic pathway, involving a crosstalk between these two pathways by the tBid protein. These death receptors may be up-regulated by a transcriptional mechanism yet unknown. Moreover, Pcy-induced apoptosis in SW620 cells involved ROS production from increased polyamine catabolism, leading to mitochondrial disruption.
REFERENCES
1. Gerhäuser C. Cancer, Chemopreventive Potential of Apples, Apple Juice, and Apple Components. Planta Med. 2008 Oct; 74 (13): 1608-1624. [ Links ]
2. Manach C, Scalbert A, Morand M, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004 May; 79 (5): 727– 747. [ Links ]
3. Mullen W, Marks SC, Crozier A. Evaluation of phenolic compounds in commercial fruit juices and fruit drinks. J Agr Food Chem. 2007 Apr; 55 (8): 3148-3157. [ Links ]
4. Guyot S, Marnet N, Drilleau J. Thiolysis-HPLC characterization of apple procyanidins covering a large range of polymerization states. J Agr Food Chem. 2001 Jan; 49 (1): 14-20. [ Links ]
5. Huemmer W, Dietrich H, Will F, Schreier P, Richling E. Content and mean polymerization degree of procyanidins in extracts obtained from clear and cloudy apple juices. Biotechnol J. 2008 Feb; 3 (2): 234–243. [ Links ]
6. Guyot S, Marnet N, Sanoner P, Drilleau JF. Direct thiolysis on crude apple materials for high-performance liquid chromatography characterization and quantification of polyphenols in cider apple tissues and juices. Method Enzymol. 2001; 335: 57-70. [ Links ]
7. Kim H, Kim EH, Eom YW, Kim WH, Kwon TK, Lee SJ, et al. Sulforaphane sensitizes tumor necrosis factor-related apoptosisinducing ligand (TRAIL)-resistant hepatoma cells to TRAILinduced apoptosis through reactive oxygen species-mediated up-regulation of DR5. Cancer Res. 2006 Feb; 66 (3): 1740-1750. [ Links ]
8. Gossé F, Guyot S, Roussi S, Lobstein AL, Fischer B, Seiler N, et al. Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis. 2005 Jul; 26 (7): 1291-1295. [ Links ]
9. Kozoni V, Tsioulias G, Shiff S, Rigas B. The effect of lithocholic acid on proliferation and apoptosis during the early stages of colon carcinogenesis: differential effect on apoptosis in the presence of a colon carcinogen. Carcinogenesis. 2000 May; 21 (5): 999–1005. [ Links ]
10. Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004 May; 14 (3): 277–287. [ Links ]
11. Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000 Apr; 256 (1): 42-49. [ Links ]
12. Gerner EW, Meyskens FL Jr. Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin Cancer Res. 2009 Feb 1; 15 (3): 758-761. [ Links ]
13. Criss WE. A review of polyamines and cancer. Turk J Med Sci. 2003 May 16; 33: 195-105. [ Links ]
14. Seiler N. Polyamine metabolism. Digestion. 1990; 46 (S2): 319–330. [ Links ]
15. Gossé F, Roussi S, Guyot S, Schoenfelder A, Mann A, Bergerat JP, et al. Potentiation of apple procyanidin-triggered apoptosis by the polyamine oxidase inactivator MDL 72527 in human colon cancer-derived metastatic cells. Int J Oncol. 2006 Aug; 29 (2): 423-428. [ Links ]
16. Duranton B, Holl V, Schneider Y, Carnesecchi S, Gossé F, Raul F, et al. Cytotoxic effects of the polyamine oxidase inactivator MDL 72527 to two human colon carcinoma cell lines SW480 and SW620. Cell Biol Toxicol. 2002 Dec; 18 (6): 381-396. [ Links ]
17. Duranton B, Holl V, Schneider Y, Carnesecchi S, Gossé F, Raul F, et al. Polyamine metabolism in primary human colon adenocarcinoma cells (SW480) and their lymph node metastatic derivatives (SW620). Amino Acids. 2003 Mar; 24 (1-2): 63-72. [ Links ]
18. Maldonado-Celis ME, Roussi S, Foltzer-Jourdainne C, Gossé F, Lobstein A, Habold C, et al. Modulation by polyamines of apoptotic pathways triggered by procyanidins in human metastatic SW620 cells. Cell Mol Life Sci. 2008 May; 65 (9): 1425-1434. [ Links ]
19. Kee K, Vujcic S, Merali S, Diegelman P, Kisiel N, Powell CT, et al. Metabolic and antiproliferative consequences of activated polyamine catabolism in LNCaP prostate carcinoma cells. J Biol Chem. 2004 Jun; 279 (26): 27050-27058. [ Links ]
20. Desidero MA, Weibel M, Mamont PS. Spermidine nuclear acetylation in rat hepatoctes and in logarithmically growing rat hepatoma cells: comparison with histone acetylation. Exp Cell Res. 1992 Oct; 202 (2): 501-506. [ Links ]
21. Inoue H, Shiraki K, Ohmori S, Sakai T, Deguchi M, Yamanaka T, et al. Histone deacetylase inhibitors sensitize human colonic adenocarcinoma cell lines to TNF-related apoptosis inducing ligand-mediated apoptosis. Int J Mol Med. 2002 May; 9 (5): 521–525. [ Links ]
22. Saunders LR, Verdin E. Ornithine decarboxylase activity in tumor cell lines correlates with sensitivity to cell death induced by histone deacetylase inhibitors. Mol Cancer Ther. 2006 Nov; 5 (11): 2777–2785. [ Links ]
23. Huerta S, Heinzerling JH, Anguiano-Hernandez Y-M, Huerta-Yepez S, Lin J, Chen D, et al. Modification of Gene Products Involved in Resistance to Apoptosis in Metastatic Colon Cancer Cells: Roles of Fas, Apaf-1, NF-kB, IAPs, Smac/DIABLO, and AIF. J Surg Res 2007 Sept; 142 (1): 184-194. [ Links ]
24. Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999 Jul; 104 (2): 155–162. [ Links ]
25. Herbeuval JP, Lambert C, Sabido O, Cottier M, Fournel P, Dy M, et al. Macrophages from cancer patients: analysis of TRAIL, TRAIL receptors, and colon tumor cell apoptosis. J Natl Cancer Inst. 2003 Apr 16; 95 (8): 611-621. [ Links ]
26. Maldonado-Celis ME, Bousserouel S, Gossé F, Minker C, Lobstein C, Raul F. Differential Induction of Apoptosis by Apple Procyanidins in TRAIL-Sensitive Human Colon Tumor Cells and Derived Trail-Resistant Metastatic Cells. J Cancer Mol. 2009 May; 5 (1): 21-30. [ Links ]
27. Chen C, Shen G, Hebbar V, Hu R, Owuor E, Kong A. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis. 2003 Aug; 24 (8): 1369–1378. [ Links ]
28. Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signaling. Curr Med Chem. 2004 May; 11 (9): 1163-1182. [ Links ]
29. Maldonado Celis, ME. Apoptotic pathways triggered by apple procyanidins in human colon adenocarcinoma cells and their derived metastatic cells. [Thesis PhD]. [Strasbourg, France]: Université de Strasbourg. 2009. 142 p.
30. Ashkenazi A. Targeting death and decoy receptors of the tumornecrosis factor superfamily. Nat Rev Cancer. 2002 Jun; 2 (6): 420-430. [ Links ]
31. Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997 Apr; 276 (5309): 111 – 113. [ Links ]
32. Kaufmann SH, Steensma DP. On the TRAIL of a new therapy for leukemia. Leukemia. 2005 Dec; 19 (12): 2195-2202. 33. Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene. 2001 Apr; 20 (17): 2122-2133. [ Links ]
34. Kreuz S, Siegmund D, Rumpf JJ, Samel D, Leverkus M, Janssen O, et al. NFkappaB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP. J Cell Biol. 2004 Aug; 166 (3): 369-380. [ Links ]
35. Rathore N, Matta H, Chaudhary PM. An evolutionary conserved pathway of nuclear factor-kappaB activation involving caspase-mediated cleavage and N-end rule pathway-mediated degradation of IkappaBalpha. J Biol Chem. 2004 Sep; 279 (38): 39358-39365. [ Links ]
36. Marini P, Denzinger S, Schiller D, Kauder S, Welz S, Humphreys R, et al. Combined treatment of colorectal tumours with agonistic TRAIL receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy: enhanced effects in vitro and dose-dependent growth delay in vivo. Oncogene. 2006 Aug; 25 (37): 5145-5154. [ Links ]
37. Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007 Oct; 13 (20): 6187-6194. [ Links ]
38. Jung EM, Lim JH, Lee TJ, Park JW, Choi KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated up regulation of death receptor 5 (DR5). Carcinogenesis. 2005 Nov; 26 (11): 1905-1913. [ Links ]
39. Kim JY, Kim EH, Park SS, Lim JH, Kwon TK, Choi KS. Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. J Cell Biochem. 2008 Dec; 105 (6): 1386-1398. [ Links ]
40. Psahoulia FH, Drosopoulos KG, Doubravska L, Andera L, Pintzas A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol Cancer Ther. 2007 Sep; 6 (9): 2591-1599. [ Links ]
41. Horinaka M, Yoshida T, Shiraishi T, Nakata S,Wakada M, Sakai T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor–related apoptosis-inducing ligand. Mol Cancer Ther. 2006 Apr; 5 (4): 945–951. [ Links ]
42. Shankar S, Chen Q, Siddiqui I, Sarva K, Srivastava RK. Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3, 4', 5 tri-hydroxystilbene): molecular mechanisms and therapeutic potential. J Mol Signal. 2007 Aug; 2 (1): 1-17. [ Links ]
43. Van Geelen CM, de Vries EG, Le TK, van Weeghel RP, de Jong S. Differential modulation of the TRAIL receptors and the CD95 receptor in colon carcinoma cell lines. Brit J Cancer. 2003 Jul; 89 (2): 363-373. [ Links ]
44. Jin Z, McDonald III ER, Dicker DT, El Deiry WS. Deficient TRAIL death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J Biol Chem. 2004 Aug; 279 (34): 35829–35839. [ Links ]
45. Maldonado-Celis ME, Bousserouel S, Gossé F, Lobstein A, Raul F. Apple procyanidins activate apoptotic signaling pathway in human colon adenocarcinoma cells by a lipid-raft independent mechanism. Biochem Bioph Res Co. 2009 Oct; 388 (2): 372-376. [ Links ]
46. Delmas D, Rébé C, Micheau O, Athias A, Gambert P, Grazide S, et al. Redistribution of CD95, DR4 and DR5 in rafts accounts for the synergistic toxicity of resveratrol and death receptor ligands in colon carcinoma cells. Oncogene. 2004 Nov; 23 (55): 8979-8986. [ Links ]
47. Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002 Nov; 277 (44): 41295-41298. [ Links ]
48. Tarahovsky YS, Muzafarov E, Kim YA. Rafts making and rafts braking: how plant flavonoids may control membrane heterogeneity. Mol Cell Biochem. 2008 Jul; 314 (1-2): 65–71. [ Links ]
49. Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000 Jun; 12 (6): 611-620. [ Links ]
50. Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999 Feb; 6 (2): 99-104. [ Links ]
51. Özoren N, El-Deiry WS. Defining caracteristics of types I and II apoptotic cell response to TRAIL. Neoplasia. 2002 Nov-Dec; 4 (6): 551-557. [ Links ]
52. Timmer JC, Salvesen GS. Caspase substrates. Cell Death Differ. 2007 Jan; 14 (1): 66-72. [ Links ]
53. Riddle-Taylor E, Nagasaki K, Lopez J, Esquivel CO, Martinez OM, Krams SM. Mutations to bid cleavage sites protect hepatocytes from apoptosis after ischemia/reperfusion injury. Transplantation. 2007 Sept; 84 (6): 778-785. [ Links ]
54. Sinicrope FA, Penington RC, Tang XM. Tumor necrosis factorrelated apoptosis-inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human colon cancer cells. Clin Cancer Res. 2004 Dec; 10 (24): 8284-8292. [ Links ]
55. Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, et al. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol Cell. 2001 Sept; 8 (3): 601-611. [ Links ]
56. Breitschopf K, Zeiher AM, Dimmeler S. Ubiquitin-mediated degradation of the proapoptotic active form of bid. A functional consequence on apoptosis induction. J Biol Chem. 2000 Jul; 275 (28): 21648-21652. [ Links ]
57. Bergmann-Leitner ES, Abrams SI. Differential role of Fas/Fas ligand interactions in cytolysis of primary and metastatic colon carcinoma cell lines by human antigen-specific CD8+ CTL. J Immunol. 2000 May; 164 (9): 4941-4954. [ Links ]
58. O'Connell J, Bennett MW, Nally K, Houston A, O'Sullivan GC, Shanahan F. Altered mechanisms of apoptosis in colon cancer: Fas resistance and counterattack in the tumor-immune conflict. Ann N Y Acad Sci. 2000 Jun; 910: 178-92; discussion 193-195. [ Links ]
59. Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, et al. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995 Feb; 373 (6513): 444-448. [ Links ]
60. Montel AH, Bochan MR, Hobbs JA, Lynch DH, Brahmi Z. Fas involvement in cytotoxicity mediated by human NK cells. Cell Immunol. 1995 Dec; 166 (2): 236-246. [ Links ]
61. Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICEinhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001 Dec; 21 (24): 8247-8254. [ Links ]
62. Cascino I, Papoff G, De Maria R, Testi R, Ruberti G. Fas/Apo-1 (CD95) receptor lacking the intracytoplasmic signaling domain protects tumor cells from Fas-mediated apoptosis. J Immunol. 1996 Jan; 156 (1): 13-17. [ Links ]
63. Arai M, Kannagi M, Matsuoka M, Sato T, Yamamoto N, Fujii M. Expression of FAP-1 (Fas-associated phosphatase) and resistance to Fas-mediated apoptosis in T cell lines derived from human T cell leukemia virus type 1-associated myelopathy/tropical spastic paraparesis patients. AIDS Res Hum Retrov. 1998 Feb; 14 (3): 261-267. [ Links ]
64. Yanagisawa J, Takahashi M, Kanki H, Yano-Yanagisawa H, Tazunoki T, Sawa E, et al. The molecular interaction of Fas and FAP-1. A tripeptide blocker of human Fas interaction with FAP-1 promotes Fas-induced apoptosis. J Biol Chem. 1997 Mar; 272 (13): 8539-8545. [ Links ]
65. Huang DC, Tschopp J, Strasser A. Bcl-2 does not inhibit cell death induced by the physiological Fas ligand: implications for the existence of type I and type II cells. Cell Death Differ. 2000 Aug; 7 (8): 754-755. [ Links ]
66. Clemons NJ, Buzzard K, Steel R, Anderson RL. Hsp72 inhibits Fas-mediated apoptosis upstream of the mitochondria in type II cells. J Biol Chem. 2005 Mar; 280 (10): 9005-9012. [ Links ]
67. Thomas LR, Johnson RL, Reed JC, Thorburn A. The C-terminal tails of Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL) and Fas Receptors have opposing functions in Fas-associated death domain (FADD) recruitment and can regulate agonist-specific mechanism of receptor activation. J Biol Chem. 2004; 279 (50): 52479-52486. [ Links ]
68. Thomas LR, Bender LM, Morgan MJ, Thorburn A. Extensive regions of the FADD death domain are required for binding to the TRAIL receptor DR5. Cell Death Diff. 2006 Dec; 13 (1): 160–162. [ Links ]
69. Trauzold A, Wermann H, Arlt A, Schütze S, Schäfer H, Oestern S, et al. CD95 and TRAIL receptor-mediated activation of protein kinase C and NF-kappaB contributes to apoptosis resistance in ductal pancreatic adenocarcinoma cells. Oncogene. 2001 Jul; 20 (31): 4258-4269. [ Links ]
Received: 04 May 2010
Accepted: 04 August 2010