Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.18 no.1 Medellín Jan./Apr. 2011
PHARMACOLOGY AND TOXICOLOGY
ANTI-INFLAMMATORY AND ANTIOXIDANT ACTIVITIES OF Phenax rugosus (POIR.) WEDD AND Tabebuia chrysanta G. NICHOLSON
ACTIVIDAD ANTIINFLAMATORIA Y ANTIOXIDANTE DE Phenax rugosus (POIR.) WEDD Y Tabebuia chrysanta G. NICHOLSON
Luis F. OSPINA G.1*; Diana M. ARAGÓN N.1; Nadezdha E. VERGEL1; Gustavo ISAZA M.2; Jorge E. PÉREZ C.2
1 Departamento de Farmacia. Facultad de Ciencias. Universidad Nacional de Colombia. Bogotá, Colombia.
2 Departamento de Ciencias Básicas. Facultad de Ciencias para la Salud. Universidad de Caldas. Manizales, Colombia.
* Corresponding author: lfospinag@unal.edu.co.
Received: 28 September 2010
Accepted: 03 March 2011
ABSTRACT
Inflammation is a multifactorial and highly regulated process associated to several chronic and degenerative diseases. Due to the fact that many inflammatory processes are sometimes characterized by oxidative stress, it has been demonstrated that antioxidants are useful as anti-inflammatory agents. Phenax rugosus (Poir.) Wedd and Tabebuia chrysanta G. Nicholson species are used in the Colombian region called ''Eje Cafetero'' due to their antiinfeccious and immunomodulatory properties. In vivo anti-inflammatory and in vitro antioxidant properties of methanolic and aqueous extracts of the previously mentioned plant species were evaluated in this research. Two acute inflammation models were used for such purpose: TPA-induced mouse ear oedema and carrageenan-induced rat paw oedema. In vitro hydroxyl, peroxyl and superoxide scavenging activity of the extracts was evaluated. The aqueous extract of Phenax rugosus was found to be promising due to its anti-inflammatory and antioxidant activities. Results obtained from this research are a support for the ethnopharmacological use of these plant species.
Key words: Phenax rugosus, Tabebuia chrysantha, reactive oxygen species, inflammation.
RESUMEN
La inflamación es un proceso multifactorial y altamente regulado que acompaña a una gran variedad de patologías crónicas y degenerativas. Dado que muchos procesos inflamatorios crónicos se acompañan de estrés oxidativo, se ha demostrado que extractos y/o compuestos captadores de radicales libres son eficaces como antiinflamatorios. Las especies Phenax rugosus (Poir.) Wedd y Tabebuia chrysanta G. Nicholson son empleadas en la región cafetera colombiana y se les atribuyen propiedades antiinfecciosas e inmunomoduladoras. En el presente estudio se evaluaron las propiedades antiinflamatorias in vivo y antioxidantes in vitro de los extractos acuosos y metanólicos de estas dos especies vegetales. Se emplearon dos modelos de inflamación aguda: edema auricular inducido por TPA en ratón y edema plantar inducido por carragenina en ratas. La actividad antioxidante in vitro se evaluó frente a los radicales hidroxilo, peroxilo y superóxido. El extracto acuoso de Phenax rugosus resultó promisorio por sus actividades antiinflamatoria y antioxidante. Estos resultados contribuyen a dar un soporte científico al uso etnofarmacológico de las dos especies vegetales estudiadas.
Palabras clave: Phenax rugosus, Tabebuia chrysantha, especies de oxígeno reactivas, inflamación.
INTRODUCTION
Besides the product offer from the pharmaceutical market, there are many other drug products with anti-inflammatory properties. Medicinal plants are still an alternative to the treatment of pathologies that include an inflammatory process. Moreover, the great Colombian plant biodiversity and the existing ethnopharmacological information motivates the research in the phytopharmacological field with the aim of contributing to the knowledge about our medicinal plants.
An enhanced yield of reactive oxygen species -ROS (i.e. superoxide radical, hydrogen peroxide and hypochlorous acid) by phagocitic cells like polimorphonuclear leucocytes (PMNs) has been demonstrated in the acute inflammatory process (1). The magnification of this process leads to an oxidative stress that injuries the inflammatory focus, which is why the antioxidant agents contribute to inhibit this important phase in the inflammatory process (2).
Phenax rugosus (Poir.) Wedd (Urticaceae) known as ''esparietaria'' and Tabebuia chrysanta G. Nicholson (Bignoniaceae) known as ''guayacán amarillo, cañahuate'' species are used in the Eje Cafetero due to its anti-infectious and immunomodulatory properties (3-5). The antiinflamatory activity of other species of the Tabebuia genus has been demonstrated by means of in vivo and in vitro studies (6). Lactone-like and lignin-like compounds with in vitro activity against HIV have been found in Phenax rugosus (Poir.) Wedd (7).
The anti-inflammatory and antioxidant activities of two extracts of Phenax rugosus (Poir.) Wedd and Tabebuia chrysanta G. Nicholson were evaluated in this research to contribute to the knowledge of their pharmacological properties.
MATERIALS AND METHODS
Plants
Phenax rugosus was collected at 1,400 m.a.s.l. and at an average temperature of 22ºC in the rural area surrounding Cartago, Valle del Cauca, Colombia. Tabebuia chrysantha was collected at 1,800 m.a.s.l. and at an average temperature of 20ºC in the rural area surrounding Palestina, Caldas, Colombia. Samples of both species were identified and classified at the herbarium of the Faculty of Agricultural Sciences in the Universidad de Caldas. Then, Phenax rugosus (Poir.) Wedd and Tabebuia chrysantha G. Nicholson samples were properly compared to the reference samples 1016 and 1525 respectively.
Extracts
Leaves were washed with abundant water, and then they were dried at 40ºC for 24 hours. Dried leaves were powdered and stored in dark glass containers at -4ºC. Aqueous and methanolic extracts were obtained from each plant by maceration. The methanolic extract was rotaevaporated, while the aqueous extract was freeze dried. Extracts were identified as follows: PA (Aqueous extract of Phenax rugosus), PM (Methanolic extract of Phenax rugosus), TA (Aqueous extract of Tabebuia chrysantha) and TM (Methanolic extract of Tabebuia chrysantha).
Phytochemical evaluation
Extracts were analyzed using common chemical tests in order to detect secondary metabolites. Alkaloids were detected with Dragendorff, Valser, Mayer and Reineckatto precipitation reagents. Flavonoids were evaluated by means of the Shinoda test; steroids and triterpenoids through the Liebermann-Burchard reaction; tannins by means of a gelatine-salt reagent; saponins through the haemolysis test; and coumarins by means of a ferric hydroxamate reaction (8).
Inflammation tests
Animals
Male Wistar rats (10-12 weeks old) weighing about 200 g were used for paw oedema and the ear oedema was performed on male Swiss ICR mice (11-13-weeks old) weighing 34-40 g. The animals were grown and housed at the Pharmacy Department under the following controlled conditions: 12 h light/darkness cycles, 21ºC of temperature, water and food ad libitum. All experiments were performed according to the ethical considerations of animal care and use (9).
Paw oedema
As we have proceeded in previous studies (10), the inflammation response wasinduced by injecting 0.05 mL of carrageenan λ (Sigma) (3% w/v in physiological solution) into the subplantar region in the right hind paw. Thirty minutes before giving the carrageenan injection to the animals, plant extracts (500 mg/kg) and indomethacine (10 mg/kg, as a positive control) were given to them through i.p. route The control group was given saline solution (1 mL/ 100 g). Each group consisted of six animals. The volume of the paws was measured with an Ugo Basile® plethysmometer 1, 3 and 5 hours after injecting the carrageenan solution. The antiinflammatory activity was expressed according to the following equation:
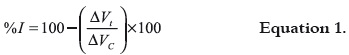
Where, ΔVt: delta of the paws' volume in the treated groups, and ΔVC: delta of the paws' volume in the control group.
Ear oedema
The TPA-induced oedema was performed on mice by methods commonly used in our research group (11). Briefly, 2.5 µg of phorbol 12-myristate 13-acetate (TPA, Sigma) were dissolved in 20 mL of acetone and, then, topically applied on the right ear of each mouse (n= 6 mice per group). Extracts (1mg/ear) and indomethacine (1mg/ear) were dissolved in acetone and topically applied on the right ear of mice immediately before the TPA was administered. The control group was treated with acetone on the right ear. Four hours after the inflammatory agent was administered, the animals were sacrificed by cervical dislocation. Circular sections (7-mm diameter) were cut from right and left ears. The sections were weighted and then antiinflammatory activity was expressed by means of the following equation:
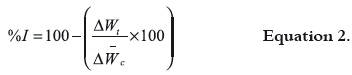
Where, ΔWt: delta of the ears' weight in the treated groups, and ΔWC: delta of the ears' weight in the control group.
Antioxidant activity
Hydroxyl radical scavenging
The ·OH scavenging activity was evaluated by deoxyribose assay, where the hydroxyl radical is generated by means of a simulation of Fenton reaction (12, 13).
The scavenging test was carried out according to Soobrattee et al. with minor modifications (14). The reagents were added in the following order: 10 μL of plant extracts (10 mg/mL), 100 μL of deoxyribose (Sigma, 28 mM), 10 μL of ascorbic acid (Sigma, 5 mM), 780 μL of buffer KH2PO4-KOH (pH 7.4, 10 mM), 50 μL of FeCl3 (400 μM in EDTANa2 400 μM), 50 μL of H2O2 (Merck, 28.4 mm). Dimethyl sulfoxide (Sigma, 2 M) was used as reference scavenger. Reaction mixtures were incubated at 37ºC for 60 minutes, followed by the addition of 1mL of trichloroacetic acid (Merk®) at 2.8% and 1mL of thiobarbituric acid (Sigma®) at 1%. The mixtures were immediately incubated at 92ºC for 15 minutes, and then they were cooled down in an ice bath for 5 minutes. The absorbance was measured at 532 nm. The same procedure was made in the series of blanks, but FeCl3 and H2O2 were avoided. Extracts were dissolved in methanol. Results were expressed as the percentage of the hydroxyl radical scavenging.
In order to dismiss a possible pro-oxidizating effect of the extracts, a control experiment was carried out simultaneously, using the same procedure but without ascorbic acid.
Peroxyl radical scavenging
The ABAP/Lysozyme method was used, in which the thermal decomposition of the ''azostarter'' 2,2'-azobis(2 methyl-amidine propane) (ABAP) produces peroxyl radical (15).
The reagents were added in the following order: 10 μL of the extract (10 mg/mL) or propylgallate (Sigma®, 10 mM), 900 μL of lysozyme (Sigma®, 55.500 U/mL), 100 μL of ABAP (Sigma®, 100 mM). The reaction mixture was incubated at 45ºC for 90 minutes and then cooled down in an ice bath. 50 μL of this reaction mixture were taken and added to 950 μL of a Micrococcus lysodeikticus (Sigma®) suspension (0.6 mg/mL dissolved in Dulbecco's buffer). The absorbance change was immediately measured at 450 nm, every 10 seconds for 30 seconds. Results were expressed in terms of percentages of inhibition of the lysozyme. Extracts were dissolved in methanol, and the other reagents were dissolved in phosphate buffer 50 mM pH 7.4.
Superoxide radical scavenging
The superoxide radical was generated by the action of the xanthine oxidase on the hypoxanthine. It produced a NBT reduction (nitroblue tetrazolium), generating a diformazan compound (12).
Reagents were added in the following order: 10 μL of extracts (10mg/mL) or n-propylgallate (Sigma®, 10 mM/mL), 100 μL of EDTANa2 (Sigma®, 10 mM), 10 μL of hypoxanthine (Sigma®, 10 mM), 100 μL of NBT (Sigma®, 1 mM), and phosphate buffer pH 7.4 (50 mM) until completing 1.2 mL of reaction mixture. Then, 100 μL of xanthine oxidase (Sigma®, 0.66 U/mL) were immediately added, and the absorbance change was measured every 10 seconds for 2 minutes at 560 nm.
Statistical analysis
For each test, results were expressed as the mean of the values ± the standard deviation. The obtained data were subjected to an analysis of simple variance (ANOVA), followed by the analysis of multiple comparisons (Tukey's test). Suppositions of normality, homogeneity, and independence of variances were previously verified.
RESULTS AND DISCUSSION
Composition of secondary metabolites
By preliminary phytochemical analysis of the extracts, positive test was obtained for: PA: Saponins, coumarins and/or terpenic lactones; PM: Saponins, coumarins and/or terpenic lactones, steroids and/or triterpenoids; TA: Alkaloids, tannins, saponins, coumarins and/or terpenic lactones; TM: Alkaloids, tannins, saponins, coumarins and/ or terpenic lactones, steroids and/or triterpenoids. These data coincide with the data reported for other species of Tabebuia genus (16-18).
Anti-inflammatory effect
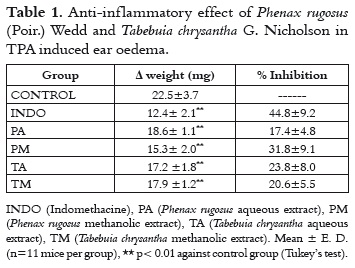
TPA induced ear oedema is useful for the in vivo evaluation of possible cyclooxygenase pathway inhibitors, due to the fact that TPA induces the liberation of arachidonic acid, increasing PGE2 production in the inflammatory focus.
In table 1, it is shown that all the evaluated extracts presented a significant anti-inflammatory effect in the TPA induced ear oedema, with inhibition levels from 17% to 32%. The PM extract showed a better anti-inflammatory effect. Methanolic extracts have steroids and/or triterpenoids, which increase the lypophilic character of the PM extract. The possible active compounds can be better absorbed through the skin of the ear of the animal. This fact would allow a better anti-inflammatory effect that the one presented by aqueous extracts. Furthermore, it is important to say that aqueous extracts are not dissolved appropriately in the vehicle commonly used in this test, which is why it is difficult to detect their possible anti-inflammatory effect in this animal assay.
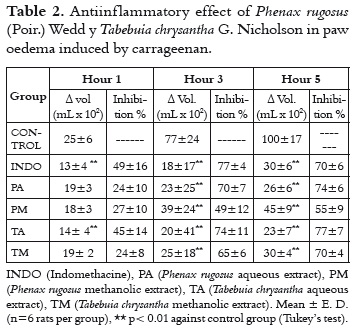
The paw oedema induced by the carrageenan is a two-phase inflammation model. The initial phase is characterized by the liberation of mediators like bradykinin, which produces a maximum of inflammation at around the third hour. In the second phase, important quantities of prostanglandins and lytic enzymes are released. Most of AINEs-like anti-inflammatory agents can be detected in this last phase (19).
Carrageenan induced paw oedema is an useful model to evaluate compounds with anti-inflammatory activity when they are administered by oral or parenteral route (i.e intraperitoneal route). As it is shown in table 2, all extracts significantly inhibit the development of the inflammation after three hours of carrageenan administration. The TA extract is able to inhibit the oedema from the first hour. This early effect is not common in vegetable extracts, and it could be related with a possible differential mechanism against the other evaluated extracts. As it was previously mentioned, the initial phase is influenced by the release of vasoactives mediators like kinines, histamine and serotonine. Also, in this early phase, there are important leukocyte migrations toward the inflammatory focus (19). This fact suggests that the TA extract participates in mechanisms related to the initial mediators of the acute inflammation, besides a possible AINE-like mechanism shared with the other extracts. This observation should be approached in mechanistic studies. The anti-inflammatory activity of other Tabebuia species has been evaluated in vivo and in vitro tests. These studies suggest mechanisms related to arachidonic acid pathway inhibition (6, 20). For the Phenax rugosus species, an anti-inflammatory effect has been reported on the oedema produced by the bite of the Bothrops asper snake (21).
The anti-inflammatory activity found in these plants coincides with the activity reported for other species with similar secondary metabolites. Recent studies have demonstrated the anti-inflammatory activity of compounds such as saponins, tannins, flavonoids, and alkaloids (22-24).
When these same extracts were administered via gavage (data not shown), they didn't show any antiinflammatory effect on the paw oedema induced by carrageenan. It is possible that the metabolism of first-step and other pre-systemic effects doesn't allow appropriate plasmatic levels of the extract's anti-inflammatory compounds.
Antioxidant activity
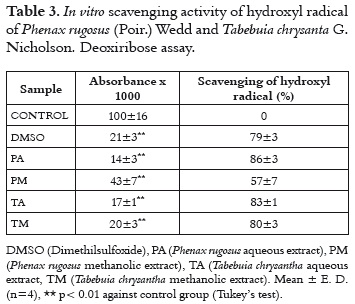
The antioxidant activity against hydroxyl radical was evaluated by deoxyribose assay. In this reaction, the reduced iron (by the ascorbate presence) reacts to hydrogen peroxide producing the ·OH. This radical promotes the degradation from deoxyribose to malonyldialdehyde, which can be detected by addying thiobarbituric acid; this addition produces a pink cromophore (12). In table 3, it is shown that all extracts possess a significant scavenging effect of the hydroxyl radical (between 57% and 86%). In a parallel experiment in absence of ascorbate (data not shown), it was detected that the TA and TM extracts could also mediate through a reducing effect the production of hydroxyl radical. In spite of this effect these extracts possess a major hydroxyl scavenging effect. The PA extract presents less interference in this sense, and it presents an excellent scavenging activity of the hydroxyl radical (86%). It is very important to find scavenging agents for the hydroxyl radical, because there are no antioxidant enzymatic systems that attenuate the effect of this radical on oxidative stress processes (13).
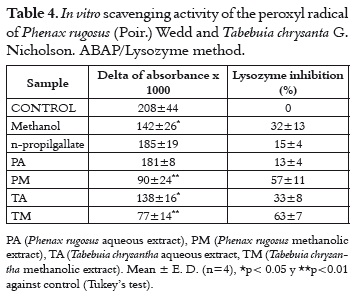
The peroxyl radical inhibits the lysozyme enzyme and, depending on the damage caused to this enzyme, it would be possible to know if a compound does or does not scavenge these radicals. Thus, the residuary lysozyme activity on Micrococcus lysodeikticus is an indicative of the damage of the enzyme by peroxyl radical (12, 25).
In table 4, data shows that only the PA extract protects lysozyme from the damage by the peroxyl radical. In a parallel experiment (data not shown), we found that the enzymatic activity of the lysozyme decreases 20% in presence of the PA extract. This apparent inhibitory effect is not very important for the scavenging assay, it only presents a light interference like false negative when interpreting the antioxidant capacity of the PA extract.
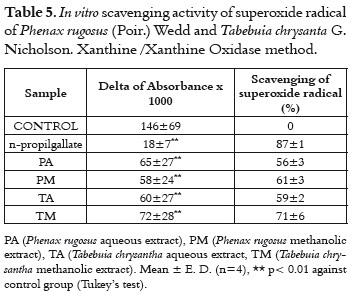
In the generation / scavenging test of the super-oxide radical, the scavengers of this radical inhibit the NBT reduction. The test evaluates the possible interference of extracts on the xanthine oxidase, this enzyme generates the superoxide radical (12).
In a control experiment (data not shown), it was demonstrated that the evaluated extracts do not inhibit the xanthine oxidase activity. In table 5, it is shown that all extracts were able to scavenge the superoxide radical more than 50%, specially the TM extract. In spite of superoxide dismutase constituting the first line of the antioxidant defence system in situations of oxidative stress, it is advantageous to have additional scavenging agents such as vegetable extracts. It is worth taking into account the importance of scavenging the superoxide radical, because this radical is the first ROS that takes place in the molecular oxygen reduction cascade. Thus, this radical is the source for the formation of other species that also participate in oxidative stress processes (26).
In summary, the four evaluated extracts present scavenging activity at least against one of the free radicals evaluated. Aqueous extracts present a better scavenging activity than methanolic extracts. The PA extract also presents a better anti-inflammatory effect in the carrageenan induced paw oedema. As it has been previously demonstrated, inflammation is characterized by the activation of phagocytic cells that synthesize and liberate a great quantity of ROS (26). For this reason, scavenging a free radical represents a viable anti-inflammatory action mechanism for a large quantity of compounds (27), especially the compounds from natural sources. This fact has been demonstrated for several vegetable species (28-31).
CONCLUSIONS
In conclusion, aqueous and methanolic extracts of the Phenax rugosus (Poir.) Wedd and Tabebuia chrysantha G. Nicholson species present in vivo anti-inflammatory activity and in vitro antioxidant activity. Further studies are required for researching on the anti-inflammatory action mechanisms and elaborating on the profile of the antioxidant activity in cases of in vivo oxidative stress. The results of this research contributed to the pharmacological knowledge of the two vegetable species studied.
REFERENCES
1. Sethi S, Dikshi M. Modulation of polymorphonuclear leukocytes function by nitric oxide. Thrombosis Res. 2000 Nov 1; 100 (2): 223–247. [ Links ]
2. Halliwell B. Reactive oxygen species in living systems: source, biochemistry and role in human diseases. Am J Med. 1991 Sept 30; 91 (3 Sup 3C): S14-S18. [ Links ]
3. Pérez JE, Isaza G, Bueno JG, Arango MC, Hincapié BL, Nieto AM, et al. Efecto de los extractos de Phenax rugosus, Tabebuia chrysantha, Althernantera williamsii y Solanum dolichosepalum sobre el leucograma y la producción de anticuerpos en ratas. Rev Med Risaralda. 2004 Nov; 10 (2): 13-21. [ Links ]
4. Álvarez ME, Isaza G, Acosta S, Yepes AG. Atividad antimicótica de Phenax rugosus (LAM) PERS y Baccharis trinervis (SW) Wedd. Biosalud. 2005 Jan-Dec; 4: 38-45. [ Links ]
5. Pérez JE, Isaza G, Acosta S. Actividad antibacteriana de extractos de Phenax rugosus y Tabebuia chrysantha. Biosalud. 2007 Jan-Dec; 6: 59-68. [ Links ]
6. Byeon SE, Chung JY, Lee YG, Kim BH, Kim KH, Cho JY. In vitro and in vivo anti-inflammatory effects of taheebo, a water extract from the inner bark of Tabebuia avellanedae. J Ethnopharmacol. 2008 Sept 2; 119 (1): 145–152. [ Links ]
7. Piccinelli AL, Mahmood N, Mora G, Poveda L, De Simone F, Rastrelli L. Anti-HIV activity of dibenzylbutyrolactone-type lignans from Phenax species endemic in Costa Rica. J Pharm Pharmacol. 2005 Sept; 57 (9): 1109-1115. [ Links ]
8. Sanabria A. Análisis fitoquímico preliminar. Metodología y su aplicación en la evolución de 40 plantas de la familia Compositae. Bogotá, Colombia: Universidad Nacional de Colombia, Facultad de Ciencias, Departamento de Farmacia; 1983. p. 58-80. [ Links ]
9. Mrad A, Cardozo CA. Utilización de animales de laboratorio en la experimentación biológica. Bogotá, Colombia: Instituto de Biotecnología, Universidad Nacional de Colombia; 2000. 277 p. [ Links ]
10. Aragón DM, Vergel NE, Ospina LF, Martínez F, Rosas JE. Efecto de naproxeno microencapsulado en microesferas de ácido poli (láctico-co-glicólico) sobre edema plantar inducido por carragenina en ratas. Vitae. 2010 Ene-Abr; 17 (1): 59-65. [ Links ]
11. Franco LA, Matiz GE, Calle J, Pinzón R, Ospina LF. Actividad antinflamatoria de extractos y fracciones obtenidas de cálices de Physalis peruviana L. Biomédica. 2007 Ene-Mar; 27 (1): 110-115. [ Links ]
12. Halliwell B. Antioxidant characterization. Methodology and mechanism. Biochem Pharmacol. 1995 May 17; 49 (10): 1341-1348. [ Links ]
13. Aruoma OI. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res. 2003 Feb-Mar; 523-524: 9-20. [ Links ]
14. Soobrattee MA, Neergheen VS, Luximon-Rammaa A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat Res. 2005 Nov 11; 579 (1-2): 200-213. [ Links ]
15. Lissi EA, Clavero N. Inactivation of lysozyme by alkylperoxyl radicals. Free Radical Res. 1990 May; 10 (3): 177-184. [ Links ]
16. Kreher B, Lotter H, Cordell GA, Wagner H. New furanonaphthoquinones and other constituents of Tabebuia avellanedae and their immunomodulating activities in vitro. Planta Med. 1988 Dec; 54 (6); 562-563. [ Links ]
17. Warashina T, Nagatani Y, Noro T. Further constituents from the bark of Tabebuia impetiginosa. Phytochemistry. 2005 Mar; 66 (5): 589-597. [ Links ]
18. Pereira EM, Machado TB, Leal IC, Jesus DM, Damaso CR, Pinto AV, et al. Tabebuia avellanedae naphthoquinones: activity against methicillin-resistant staphylococcal strains, cytotoxic activity and in vivo dermal irritability analysis. An Clinical Microbiol Antimic. 2006 Mar 22; 5 (1): 5-7. [ Links ]
19. Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenan oedema in rats. J Pharmacol Exp Ther. 1969 Mar; 166 (1): 96-103. [ Links ]
20. Koyama J, Moritaa I, Tagahara K, Hira K. Cyclopentene dialdehydes from Tabebuia impetiginosa. Phytochemistry. 2000 Apr; 53 (8): 869-872. [ Links ]
21. Badilla B, Chaves F, Córdoba F, Guadamuz L, Mora G, Poveda LJ. Inhibition of edema-forming and hemorrhagic activities of Bothrops asper snake venom by Phenax angustifolius and Phenax rugosus (Urticaceae) extracts. Pharmacognosy Magazine. 2005 Oct-Dec; 1 (4): 159-164. [ Links ]
22. Sparg SG, Light ME, van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol. 2004 Oct; 94 (2-3): 219-243. [ Links ]
23. Barbosa-Filho JM, Piuvezam MR, Moura MD, Silva MS, Batista Lim KV, Leitão da-Cunha EV, et al. Anti-inflammatory activity of alkaloids: A twenty-century review. Brazilian J Pharmacognosy. 2006 Jan-Mar; 16 (1): 109-139. [ Links ]
24. Fawole OA, Ndhlala AR, Amoo SO, Finnie JF, van Staden J. Anti-inflammatory and phytochemical properties of twelve medicinal plants used for treating gastro-intestinal ailments in South Africa. J Ethnopharmacol. 2009 Jun 22; 123 (2): 237-243. [ Links ]
25. Loghin F, Chagraoui A, Asencio M, Comoy E, Speisky H,Cassels BK, et al. Effects of some antioxidative aporphine derivatives on striatal dopaminergic transmission and on MPTP-induced striatal dopamine depletion in B6CBA mice. Europ J Pharm Sci. 2003 Feb; 18 (2): 133-140. [ Links ]
26. Halliwell B, Gutteridge MC. Free Radicals in Biology and Medicine. Oxford, UK: Clarendon Press Oxford; 2007. 689p. [ Links ]
27. Conner EM, Grisham MB. Inflammation, free radicals and antioxidants. Nutrition. 1996 Apr; 12 (4): 274-277. [ Links ]
28. Gonçalves C, Dinis T, Batista MT. Antioxidant properties of proanthocyanidins of Uncaria tomentosa bark decoction: a mechanism for anti-inflammatory activity. Phytochemistry. 2005 Jan; 66 (1): 89-98. [ Links ]
29. Tunalier Z, Kosar M, Kupeli E, Calis I, Baser K. Antioxidant, anti-inflammatory, anti-nociceptive activities and composition of Lythrum salicaria L. extracts. J Ethnopharmacol. 2007 Apr 4; 110 (3): 539-547. [ Links ]
30. Backhouse N, Rosales L, Apablaza C, Golty LE, Erazo S, Negrete R, et al. Analgesic, anti-inflammatory and antioxidant properties of Buddleja globosa, Buddlejaceae. J Ethnopharmacol. 2008 Mar 5; 116 (2): 263-269. [ Links ]
31. Conforti F, Sosa S, Marrelli M, Menichini F, Statti GA, Uzunov D, et al. In vivo anti-inflammatory and in vitro antioxidant activities of mediterranean dietary plants. J Ethnopharmacol. 2008 Feb 28; 116 (1): 144-151. [ Links ]