Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.18 no.1 Medellín Jan./Apr. 2011
PHARMACEUTICAL INDUSTRY
EVALUATION OF THE AVOBENZONE PHOTOSTABILITY IN SOLVENTS USED IN COSMETIC FORMULATIONS
EVALUACION DE LA FOTOESTABILIDAD DE LA AVOBENZONA EN SOLVENTES USADOS EN FORMULACIONES COSMÉTICAS
Jhon Jairo VALLEJO1; Monica MESA2; Cecilia GALLARDO1*
1 Facultad de Quimica Farmacéutica, Universidad de Antioquia Calle 67 Nº 53-108, Bloque 2, Medellín, Colombia.
2 Instituto de Quimica FCEN. Universidad de Antioquia Calle 67 Nº 53-108, Bloque 2, Medellín, Colombia.
* Corresponding author: cgallardo@farmacia.udea.edu.co.
Received: 01 March 2010
Accepted: 06 September 2010
ABSTRACT
The use of sunscreen products is very important nowadays. The photostability of UV filters incorporated in these products must be preserved during its storing and application processes in order to achieve the claims about photoprotection of the commercial products. The photostability of avobenzone, one of the most common UVA filters in commercial sunscreens, is assessed in solvents intended for cosmetic formulations. The study is carried out by following the spectral behavior of avobenzone solutions under irradiation and after storing them in the dark. Different photobehaviors of the avobenzona are found in the selected solvents, some of which can be explained in the same way than in analytical solvents; but for others, the structural and physical properties of the solvents must be taken into account. The most appropriate solvents for ensuring the photostability of avobenzona are mineral oil and isopropyl myristate.
Key words: Avobenzone, UV photoradiation, photostability, photoisomerization, UV filters.
RESUMEN
El uso de antisolares es muy importante en la actualidad. La fotoestabilidad de los filtros UV usados en estos productos se debe conservar durante su almacenamiento y aplicación, con el fin de cumplir su papel fotoprotector. La fotoestabilidad de la avobenzona, uno de los filtros UVA más usados en la preparación de antisolares comerciales, es evaluada, por primera vez, en solventes cosméticos. Este estudio es llevado a cabo siguiendo el comportamiento espectral de soluciones de avobenzona cuando es sometida a irradiación, y luego de su almacenamiento en la oscuridad. La avobenzona presenta un comportamiento diferente en cada solvente, en algunos casos este comportamiento se puede explicar en la misma forma que en los solventes grado analítico, pero en otros se deben tener en cuenta las propiedades estructurales y fisicas del solvente. Los solventes donde la avobenzona es fotoestable son el aceite mineral y el miristato de isopropilo.
Palabras clave: Avobenzona, fotoradición UV, fotoestabilidad, fotoisomerización, filtros UV.
INTRODUCTION
Nowadays, people have increased their awareness about the harmul effects of UV radiation on human skin (1), thus sunscreen products are not cosmetics being used only for vacations activities. These products are now also included in the daily routine; people are using them every day of the year, in order to preserve the health of their skin in the short and the long term.
Sunscreen products contain inorganic compounds with a high refractive index acting as a physical barrier and/or UV filters, which activity is due to the ability of absorbing the UV radiation. UVB filters had been the most used type of filters in this kind of products because of the previous conception that only the radiation in the spectral range beetwen 290 nm and 320 nm (UVB) was dangerous for the human skin. But when it was demostrated that UVA radiation (320 nm-400 nm) also produces skin damages such as photocarcinogenesis and photoaging, UVA filters were also included in sunscreen products. Nowadays, there are broad-spectrum filters available that provide protection from UVB and UVA radiation (2, 3), and the development of new filters is a field of intensive research. Nevertheless, since these new broad-spectrum filters are more expensive, most of the sunscreen formulations combine only UVA and UVB filters (4).
The high capability of UV filters to absorb UV energy must be maintained for a long period of time in order to achieve the photoprotection claim expected for commercial sunscreen products. However, the excited molecules formed by absortion of UV radiation return to the basal state by different radiative and non-radiative decay mechanisms (5). Some of these mecanisms can affect their activity, leading to the formation of new compounds by photoaddition, substitution, cycloaddition, isomerization, photofragmentation reactions, etc (6, 7). These new compounds can be inactive (they do not absorb the UV radiation) or favor the degradation of biocomponents on the skin by photosensibilization, which is dangerous for the human health (8). Therefore, the photostability studies of active molecules used in sunscreen formulations are of major importance.
The most common UVA filter in the formulation of sunscreens is the 4-tert-butyl-4'-methoxydibenzoylmethane, known as Avobenzone (AVO), which can absorb a broadband UVA radiation (7) due to its highly conjugated structure. This UVA filter is commonly used in concentrations between 3.0 to 10.0%. The photochemical behavior of AVO when dissolved in analytical grade organic solvents (such as methanol, acetonitrile and hexane) has been extensively documented. And it has been found that the AVO photostability is highly dependent on the polarity and proticity of the solvent (1, 5, 9 - 15).
The objective of this research work is to analyze the effects of different commercial solvents used in cosmetic products on the AVO photostability. This study consists in measuring the decrease in absorbance in the UVA range and the recovery after stopping the irradiation. Unlike analytical grade solvents, the selected commercial solvents can offer a more complex media for the photochemistry relaxation. This is the first time that a study of AVO photostability in solvents used in cosmetic formulations is carried out, which will provide important insights about the formulation of commercial sunscreens products.
MATERIALS AND METHODS
Materials
HPLC grade acetonitrile and methanol (Merck®), PA ethanol and hexane (Merck®). Cosmetic solvents which consisted of industrial products obtained from local distributors with purity and brand name described as follows: Cyclomethycone (Cyclopentasiloxane or Decamethylcyclopentasiloxane > 60%, and Cyclohexasiloxane or Dodecamethylcyclohexasiloxane > 30%, Dow Corning®), Hydrogenated polyisobutene (purity non-reported, Basf®), Paraffinum liquidum or mineral oil (99%, SBC Oil LTDA®), Isopropyl myristate (99%, Croda®), Isopropyl palmitate (99.4%, Croda®), Decyloleate (BP, British Pharmacopoeia, Cognis®), and Octyldodecanol (90.0 Cognis®). The Avobenzone used was Parsol 1789 (DSM Nutritional Products AG®). All solvents and AVO were used without further purification.
Methods
Sample preparation
The AVO was dissolved in analytical solvents (2.00 x 10-5 M) and in cosmetic solvents (2.66 x 10-5 M). Every experiment was made in triplicate.
Irradiation
Samples were irradiated at 50°C in a SOLARBOX 1500e chamber with a xenon lamp, working at 1000 W/m2, equipped with a 280 nm outdoor filter in order to mimic the solar UV radiation that reaches the earth. The irradiation time was 14 hours for analytical solvents, and a second approach was performed in order to obtain more information about the photodegradation kinetics in analytical solvents. In this sense, the absorbance at 358 nm was monitored in a Cary 50 spectrophotometer every 2 hours for ~6 hours. In the case of cosmetic solvents, the irradiation time was 1 hour, UV spectra and absorbance in the λmax (355.1-349.9nm) were recorded every 10 min, the temperature was 50ºC. In order to avoid the evaporation of the solvents during the irradiation experiments, the samples are packed in sealed glass containers.
UV measurements
They were carried out in a Cary 50 spectrophotometer, in 1mm quartz cells. The spectra were recorded from 200 nm to 400 nm, and the absorbance was measured at 358 nm in the analytical solvents 0,6 and at λmax in the cosmetic solvents. The measurements were made immediately after samples were withdrawn from the irradiation chamber.
Absorbance recovery in the dark
After irradiation, the samples were stored under quiescent conditions in the dark in the same containers. Samples using analytical grade solvents were stored for 15 days; and the samples in cosmetic solvents were stored for 48 hours. Later, spectra and absorbance were measured using the same spectrophotometer.
The decrease % and recovery % of the absorbing capacity were calculated from the absorbance values (1) as follows:
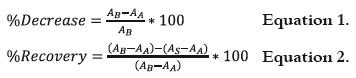
Where AB is Absorbance before irradiation, AA is Absorbance after irradiation and AS is Absorbance after storing dark.
RESULTS AND DISCUSSION
AvO photobehavior in analytical grade solvents
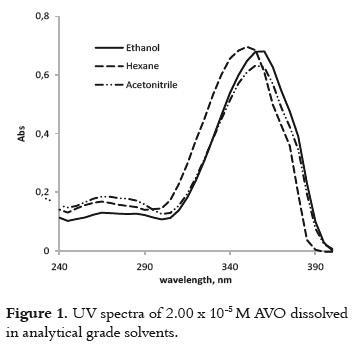
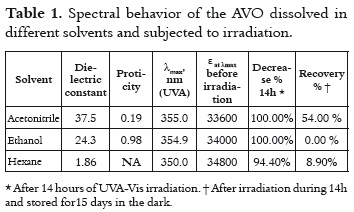
The UV spectra of the AVO that was dissolved in hexane, ethanol and acetonitrile, exhibit a peak of maximum absorption (λmax) in the UVA range, and other less intense peaks in the UVB-UVC region as is shown in figure 1. This observation is congruent with the coexistence of both diketo and enol AVO tautomers, where the enol tautomer absorbs around 355 nm and the diketo tautomer absorbs around 260 nm (10). The tautomers' relative concentrations depend on the polarity and proticity of the solvent (9, 16). The absorbance maximum of around 355nm is due to the π → π* transitions in the enol form (17). A shoulder at 370 nm is also observed, which has been ascribed to the presence of enol-trans isomer (18), while the signal at 350 nm has been attributed to the enol-cis isomer.
The bathochromic shift for the λmax, as the solvent polarity increases, confirms the π-π* transition, which is typical of enone-type molecules. This fact is shown in table 1. Since this shift is due to dipole – dipole interactions between the excited enol form and the solvent, it is expected that interactions will be stronger in the presence of acetonitrile and ethanol, which are the polar solvents with the highest dielectric constant.
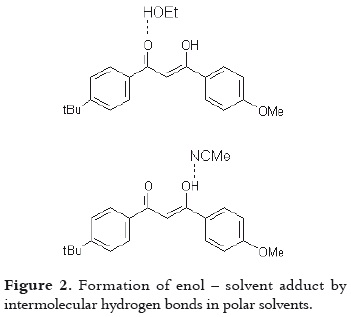
Moreover, the differences in the molar absorptivities, ε, depend on the enolization degree (19), as it is shown in table 1. The non-polar solvent allows the intramolecular hydrogen bond in this β-diketone, stabilizing the enol tautomer. And the polar solvents favor the formation of intermolecular hydrogen bonds between the AVO molecules and the solvent, decreasing the absorbance at λmax, as it is shown in figure 2. The effect of the acetronitrile on the enol/keto ratio, and the AVO absorption has been already reported (11).
The AVO photostability study was carried out in two steps: the first step consisted in the irradiation of the AVO, which was dissolved in the selected solvents; an UVA absorbance decrease was observed in this step. And the second step consisted in the absorbance recovery in the dark. The absorbance values in the UVA range, in each step, were compared with the values measured for samples before the irradiation process, and calculating the decrease % and recovery %.
The UV spectra for the AVO samples, which was recorded immediately after stopping the irradiation for a 14h period of time, showed a total absorption bleaching on the UVA region according to table 1, with a concomitant increase of the absorption on the UVC region. The samples exhibited a new maximum absorption value at 245 nm in ethanol, 250 nm in hexane, and 266 nm in acetonitrile (the spectra are not shown in this study). According to the literature, the diketo tautomer exhibits the λmax in this UVC range (10), which could indicate the enol – keto isomerization induced by radiation. However, many photoproducts also absorb radiation in this region (9) and, therefore, the photodegradation processes cannot be rejected.
An approach to the decay mechanisms in each solvent can be reached by means of the analysis of the UV spectral data recorded after storing the irradiated samples in the dark for 15 days. The results were summarized in table 1 under the Recovery % field. The high Recovery % in acetonitrile (54.0%) indicates that this solvent favors a reversible process, such as the enol – keto isomerization. In hexane and ethanol, the low Recovery % indicates an irreversible process; thus the UVC signal could be mainly due to the formation of photoproducts by a-cleavage of the keto tautomer and their subsequent reactions (12).
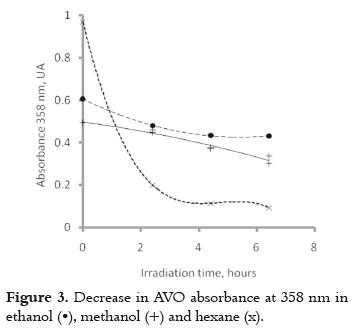
The UVA absorbance was measured during the first 6 hours of irradiation in order to collect more data for explaining the similarity among the photobehavior of the AVO dissolved in hexane and ethanol, as well as the differences on the polarity of these two solvents. Results show that the decrease in absorbance is lesser and slower in ethanol or methanol than in hexane, as it is shown in figure 3. AVO is more photostable in polar solvents, which coincides with the results found by other authors (9).
According to our results and based on the literature, some insights on the decay pathways of AVO in the analytical grade solvents studied can be summarized as follows (figure 4):
a) The aprotic polar solvent (acetonitrile) pro-motes the photoisomerization, which could be reverted in the dark (pathway 4 – 7 in figure 4). Two different mechanisms of enol – keto photoisomerization have been proposed: the first mechanism involves the formation of transient species when AVO is irradiated at 355 nm. These species are attributed to the enol Z isomers, they are formed by proton interchange, and they are in equilibrium with the enol E isomers, the chelated enol and the trans-diketo tautomers (10, 20). The second mechanism, proposed recently by Mturi G. J et al (9), involves the formation of a adduct between the non-chelated enol tautomer and the solvent by means of an intemolecular hydrogen bond. The excited state of this adduct leads to the formation of enolate ion that reacts with O2(1Δg), producing an enol radical and subsequently the keto isomer. The formation of solvent – AVO adduct is an important step in this latter mechanism.
b) The photochemical behavior of AVO in polar and protic solvents includes intramolecular hydrogen bond cleavage (pathway 4-10-9-8 in Figure 4), and photodegradation (pathway 1- 2 in the Figure 4). Since the enol decay is very slow in ethanol or methanol, as it is shown in Figure 3, and these polar solvents favor the proton interchange, it is possible that the intramolecular hydrogen bond cleavage will be the main decay process, as it has been proposed before based on the laser flash photolysis studies in the same solvent (10).
This cleavage produces a non-chelated enol (enol Z-isomer), which returns to chelated enol as ground state, involving proton interchange (21).
c) In hexane, where AVO molecules are preferentially in the ''chelated'' enol form, the enol – keto photoisomerization is less expected according to the mechanism proposed by Mturi G. J et al (9), or by Cantrell et al (10). Therefore, the photodegradation could be the most important decay mechanism (pathway 1 – 2, in figure 4).
AvO photobehavior in solvents used in cosmetic formulations
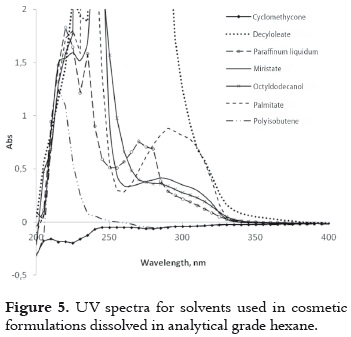
the commercial solvents selected for the second part of this research are widely used for sunscreen formulations in the cosmetic industry. Some of them show absorption on the UVB range, which must be taken into account for the study of the radiation effect on the AVO photobehavior. Since these solvents do not absorb radiation in a significative way around the λmax of AVO, as it is shown in figure 5, it is possible to follow the AVO absorption changes in the mentioned range.
Table 2 shows that AVO dissolved in the selected cosmetic solvents exhibits a maximum absorbance at λmax, between 350 nm – 355 nm before irradiation. This fact is mainly associated to the absorption by the enol tautomer, as it was previously explained with the analytical solvents. Furthermore, the change in the molar absorptivities indicates a shift on the keto – enol equilibrium when the AVO is dissolved in each kind of cosmetic solvent. These differences cannot be interpreted in a direct way in function of the dielectric constant or other properties, such as viscosity or the presence of some impurities (neither identified, nor quantified in this research). This impossibility increases the complexity of these media.
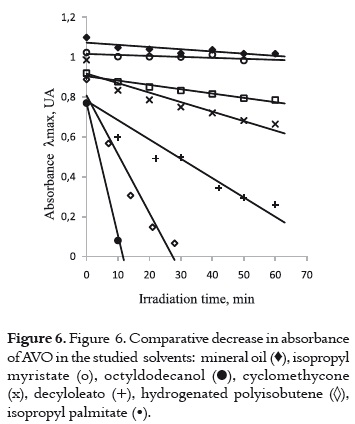
The study of the photochemical behavior of AVO in these cosmetic solvents was carried out by following the same methodology described in the analytical solvents: measuring the decrease in absorption under irradiation and the absorbance recovery after storing the irradiated samples in the dark. Since the photodegradation in this type of solvents was faster than in the analytical solvents, it was necessary to reduce the irradiation time to 1 hour, in order to observe the decrease in absorbance for each solvent. Even though all the chosen cosmetic solvents have similar dielectric constant and oleic nature, the AVO exhibits different photochemical behaviors depending on which solvent it was dissolved, according to the results shown in table 2 and in figure 6.
According to the results of the studies of absorbance decrease and recovery on the evaluated cosmetic solvents in table 2, and the rate of the decrease in absorbance in figure 6, four different behaviors were found for the AVO:
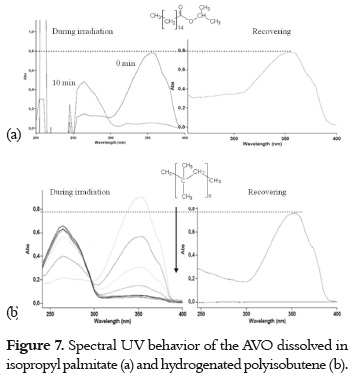
a) A high and fast UVA absorbance decrease with a concomitant increase of the absorbance at around 265 nm, and a high or even complete recovery in the dark. This fact was observed when the AVO was dissolved and irradiated in isopropyl palmitate (figure 7a) and in hydrogenated polyisobutene, as it is shown in figure 7b.
These results indicate that enol-keto photoisomerization (pathway 4 -7, in figure 4) has a higher level of importance than photodegradation in both solvents. The absorption decrease and recovery is slower and lower in hydrogenated polyisobutene than it is in isopropyl palmitate. In the former solvent, an isosbestic point on the spectral evolution during the irradiation was observed, corroborating the presence of different AVO isomers in equilibrium. The behavior of AVO on the isopropyl palmitate is similar to the behavior found in the polar solvent ethyl acetate (9), due to its hydrogen bonding acceptor character, which promotes the formation AVO – solvent adduct and, therefore, promotes photoisomerization. Photodegradation is expected in hydrogenated polyisobutene due to its non polar character. Nevertheless, the photodegradation can be prevented due to its higher viscosity and long hydrocarbon chain (compared with hexane), which could confer protection to the AVO molecules. Also, the low molar absorptivity of AVO in this solvent (table 2) could make the AVO molecules less prone to photodegradation by α-cleavage.
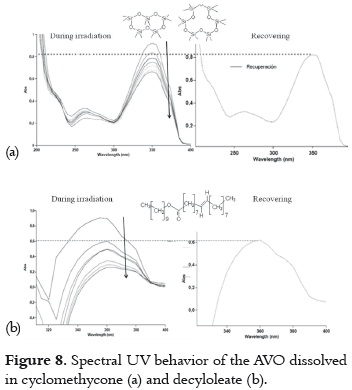
b) The UVA absorption decrease and recovery is slow and not very high. This second situation is observed when cyclomethicone or decyloleate are used, as it is shown in figure 8.
The presence of oxygen in the molecules of these solvents could confer them a hydrogen-bond acceptor nature, favoring the non-chelated enol tautomer. This tautomer could be transformed into the diketo tautomer through photoisomerization (pathway 4 -7, in the figure 4), but the low recovery level indicates that AVO also suffers from photodegradation through the α-cleavage (pathway 1-2, in figure 4).
The rate of decrease in absorbance is lesser in cyclomethicone than in decyloleate, probably due to the occlusion of the AVO molecules in the rigid and tridimensional cavities of this solvent. This fact offers a steric effect on the chelated – nonchelated enol form transitions. The unsaturation on the decyloleate molecules could give rise to oxygen reactive species, which participate on the AVO degradation.
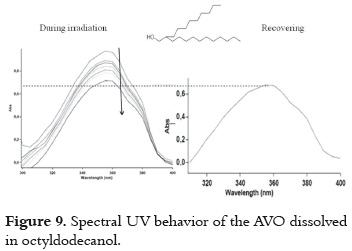
c) Low AVO absorbance decrease and minor decay rate without recovering in the dark. This behavior is observed when AVO is dissolved in octyldodecanol (figure 9).
It is possible that the photodegradation (pathway 1 – 2 in figure 4) can be involved in the decay mechanism of excited species of AVO. However, the presence of the OH group in this solvent probably favors the intramolecular hydrogen bond cleavage (pathway 8 – 10, in figure 4), as it is observed in ethanol and other alcohols (10) retarding the photodegradation process.
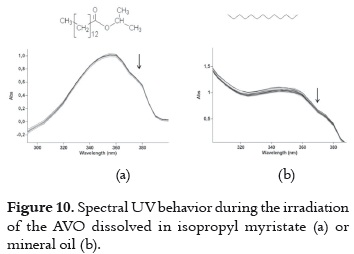
d) Very low UVA absorption decrease with the smallest decay rates. When AVO is dissolved in isopropyl myristate (figure 10a) or mineral oil (figure 10b), an insignificant decrease in absorbance is observed, indicating a high photostability level of the AVO in these media. AVO photoisomerization and photodegradation are avoided in these solvents, and they are not expected due to the chemical nature of these two solvents. Thus, it is not possible to explain such behavior only in function of the chemical properties. Probably, the impurities and viscosity may be involved.
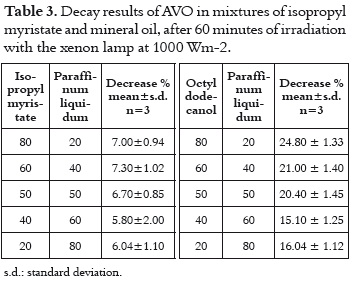
These two solvents were evaluated together taking into account that they offer the best media for having a very photostable AVO, and that they constitute a common mixture on the cosmetic formulation due to the differences on their polarity. The decay in these samples does not vary in function of the proportion of the two solvents. The difference between the average absorption decay in this mixture (6.57%, shown in table 3) with those values measured in each solvent (7.84% and 4.52% in mineral oil and isopropyl myristate, respectively) is not important, because they show that even though there is not a synergic effect, the mixture does not have a deleterious effect on the photostability.
When the mineral oil is mixed with a solvent in which the AVO absorption decay is slow, such as octyldodecanol, the photostability of the AVO increase as the contribution of the mineral oil is higher, showing that the contribution of a second solvent does not represent any importance.
CONCLUSIONS
The photochemistry of AVO is a complex process, not only by the presence of several isomers in an excited state, but also because the decay mechanisms are strongly dependent on the solvent. The high decrease % of the absorption capacity in all the analytical grade solvents evaluated, indicates that AVO is not photostable on them because it can suffer reversible photoisomerization and/or photodegradation. The screening of different commercial solvents used in cosmetic formulations allows to identify different photobehaviors of the AVO. Some of these photobehaviors can be explained in the same way that they can be explained for analytical solvents. But for other photobehaviors, the physical properties of the solvents must be taken into account. For example, in cyclomethycone, hydrogenated polyisobutene, mineral oil and isopropyl myristate, the structure, viscosity and ability for absorbing the UVA radiation must be considered.
The main conclusion of this research work is related to the selection of the solvent that can provide photoprotection to AVO. This selection has consequences of major importance on both the cosmetic properties and the protecting effect of sunscreens. Special care must be taken with solvents in which a high decrease is observed, because the formation of photoproducts can have deleterious effects on the human health. Also, a solvent in which there is a high and faster decrease (even if it is followed by recovery) is not a good choice, because it means that the AVO will not work opportunely. The best solvent for ensuring the photoprotective promise, only must allow a minor decrease in absorbance, such as mineral oil and/or isopropyl myristate.
ACKNOWLEDGEMENTS
This research was supported by grants provided by the CODI (Commitee for Research Development) from the Universidad de Antioquia and QUIFARMA. The authors would like to thank the LEA laboratory.
REFERENCES
1. Paris C, Lhiaubet-Vallet V, Jimenez O, Trullas C, Miranda MA. A blocked diketo form of avobenzone: Photostability, photosensitizing properties and triplet quenching by a triazine-derived UVB-filter. Photochem. Photobiol. 2009 Jan-Feb; 85 (1): 178-184. [ Links ]
2. Venditti E, Spadoni T, Tiano L, Astolfi P, Greci L, Littarru G P, Damiani E. In vitro photostability and photoprotection studies of a novel 'multi-active' UV-absorber. Free Radical Bio Med. 2008 Aug 1; 45 (3): 345-354. [ Links ]
3. Moyal DD, Fourtanier AM. Broad-spectrum sunscreens provide better protection from solar ultraviolet-simulated radiation and natural sunlight-induced immunosuppression in human beings. J Am Acad Dermatol 2008 May; 58 (5 Suppl 2): S149-154. [ Links ]
4. Chaudhuri R.K., Lascu Z, Puccetti G, Deshpande A.A, Paknikar S.K. Design of a photostabilizer having built-in antioxidant functionality and its utility in obtaining broad-spectrum sunscreen formulations. Photochem Photobiol. 2006 May; 82 (3): 823-828. [ Links ]
5. Herzog B, Wehrle M, Quass K. Photostability of UV absorber systems in sunscreens. Photochem Photobiol. 2009 Jul -Aug; 85 (4): 869-878. [ Links ]
6. Turro N.J.Modern molecular photochemistry. California, EEUU: University science books; 1991. 628 p. [ Links ]
7. Bonda C, Steinberg D C. A new photostabilizer for full spectrum sunscreens. Cosmet Toil. 2000; 115 (1): 37-45. [ Links ]
8. Armeni T, Damiani E, Battino M, Greci L, Principato G. Lack of in vitro protection by a common sunscreen ingredient on UVAinduced cytotoxicity in keratinocytes. Toxicology. 2004 Oct 15; 203 (1-3): 165-78. [ Links ]
9. Mturi G J, Martincigh B S. Photostability of the sunscreening agent 4-tert-butyl-4-methoxydibenzoylmethane (avobenzone) in solvents of different polarity and proticity. J Photochem Photobiol A: Chem. 2008 Dec 15; 200 (2-3): 410-420. [ Links ]
10. Cantrell A, McGarvey D J. Photochemical studies of 4-tertbutyl-4-methoxydibenzoylmethane (BM-DBM). J Photochem Photobiol B: Biol. 2001 Nov 15; 64 (2-3): 117-122. [ Links ]
11. Dubois M, Gilard P, Tiercet P, Deflandre A, Lefebvre M.A. Photoisomerisation of the sunscreen filter PARSOL© 1789. J Chim Phys. 1998 Feb; 95 (2): 388-394. [ Links ]
12. Schwack W, Rudolph T. Photochemistry of dibenzoyl methane UVA filters, Part 1. J Photochem Photobiol B: Biol. 1995 Jun; 28 (3): 229-234. [ Links ]
13. Roscher NM, Lindemann MKO, Kong SB, Cho CG, Jiang P. Photodecomposition of several compounds commonly used as sunscreen agents. J Photochem Photobiol A: Chem. 1994 May; 80 (1): 417-421. [ Links ]
14. Dondi D, Albini A, Serpone N. Interactions between different solar UVB/UVA filters contained in commercial suncreams and consequent loss of UV protection. Photochem Photobiol Sci 2006; 5 (9): 835-843. [ Links ]
15. Huong SP, Rocher E, Fourneron J-D, Charles L, Monnier V, Bun H, et al. Photoreactivity of the sunscreen butylmethoxydibenzoylmethane (DBM) under various experimental conditions. J Photochem Photobiol A: Chem. 2008 Apr; 196 (1): 106-112. [ Links ]
16. Tobita S, Ohba J, Nakagawa K, Shizuka H. Recovery mechanism of the reaction intermediate produced by photoinduced cleavage of the intramolecular hydrogen bond of dibenzoylmethane. J. Photochem Photobiol A: Chem. 1995 Dec; 92 (1-2): 61-67. [ Links ]
17. Zawadiak J, Mrzyczek M. UV absorption and keto- enol tautomerism equilibrium of methoxy and dimethoxy 1,3-diphenylpropane-1,3-diones. Spectrochim Acta A Mol Biomol Spectrosc. 2010 Feb; 75 (2): 925-929. [ Links ]
18. Nardo L, Paderno R, Andreoni A, Másson M, Haukvik T, Tønnesen HH. Role of H-bond formation in the photoreactivity of curcumin. Spectroscopy. 2008; 22 (2-3): 187-198. [ Links ]
19. Silverstein RM, Basssler GC. Morril TC. Spectrometric identification of organic compounds. 5ª ed. EEUU: John Wiley & Sons; 1991. 419 p. [ Links ]
20. Aspée A, Aliaga C, Scaiano JC. Transient enol isomers of dibenzoylmethane and avobenzone as efficient hydrogen donors toward a nitroxide pre-fluorescent probe.Photochem Photobiol. 2007 May - Jun; 83 (3): 481-485. [ Links ]
21. Lhiaubet-Vallet V, Marín M, Jiménez O, Gorchs O, Trullasb C, Miranda M A. Filter interactions. Photostabilization, triplet quenching and reactivity with singlet oxygen. Photochem Photobiol Sci. 2010 Apr; 9 (4): 552-558. [ Links ]