Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.18 no.1 Medellín Jan./Apr. 2011
NATURAL PRODUCTS
ANTIQUORUM SENSING ACTIVITY OF ESSENTIAL OILS ISOLATED FROM DIFFERENT SPECIES OF THE GENUS Piper
ACTIVIDAD ANTIQUÓRUM SENSING DE ACEITES ESENCIALES AISLADOS DE DIFERENTES ESPECIES DEL GÉNERO Piper
Jesús T. OLIVERO V.1*; Nerlis P. PÁJARO C.1; Elena STASHENKO2
1 Environmental and Computacional Chemistry Group. Faculty of Pharmaceutical Sciences. Universidad de Cartagena. Campus of Zaragocilla. Cartagena, Colombia.
2 CENIVAM. Campus UIS. Building C 45. Bucaramanga, Colombia.
* Corresponding author: joliverov@unicartagena.edu.co.
Received: 21 April 2010
Accepted: 16 November 2010
ABSTRACT
Quorum sensing is a bacterial communication mechanism that depends on population density, and occurs through molecules called autoinducers. These molecules activate receptors, enabling the transcription of genes that encode information needed to control several biochemical mechanisms associated with bacterial survival and pathogenicity. The aim of this study is to evaluate the inhibitory effect of essential oils of three species of Piper on the production of violacein, induced by N-hexanoyl homoserine lactone in Chromobacterium violaceum CV026. Results show that essential oils from Piper bredemeyeri, Piper brachypodom and Piper bogotence present 50% inhibitory concentration (IC50) for quorum sensing of 45.6 µg/mL, 93.1 µg/mL, and 513.8 µg/mL, respectively. However, in terms of cell growth, IC50 values for these oils are greater than 1000 µg/mL. These data suggest that essential oils isolated from Piper species found in Colombian flora are good candidates for the development of antiquorum sensing molecules, with possible applications in the control of bacterial diseases.
Key words: Inhibition, quorum sensing, essential oils, genus Piper, bacterial infections.
RESUMEN
El quórum sensing es un mecanismo de comunicación bacteriana que depende de la población celular y que ocurre a través de moléculas llamadas autoinductores, las cuales activan receptores que permiten la transcripción de genes que codifican la información necesaria para controlar diversos mecanismos bioquímicos asociados con la supervivencia y la patogenicidad bacteriana. El objetivo de este trabajo consiste en evaluar el efecto inhibitorio de los aceites esenciales de tres especies del género Piper sobre la producción de violaceína inducible por N-hexanoil homoserina lactona en Chromobacterium violaceum CV026. Los resultados señalan que los aceites esenciales de Piper bredemeyeri, Piper brachypodom y Piper bogotence poseen una concentración inhibitoria 50% (CI50) para el quórum sensing de 45.6 µg/mL, 93.1 µg/mL y 513.8 µg/mL, respectivamente. Sin embargo, en términos de crecimiento celular, el valor de CI50 para estos aceites es mayor de 1000 µg/mL. Estos datos sugieren que los aceites esenciales aislados de especies de Piper encontrados en la flora Colombiana son buenos candidatos para el desarrollo de moléculas antiquórum sensing, con posibles aplicaciones en el control de enfermedades bacterianas.
Palabras clave: inhibición, quórum sensing, aceites esenciales, género Piper, infecciones bacteriana.
INTRODUCTION
Quorum sensing (QS) is a cell communication mechanism through which signal molecules called autoinducers activate specific receptors associated with transcription signals for controlling various biochemical processes. Some of these processes are biofilm formation, expression of virulence factors, luminescence, pigment production, and mechanisms of resistance to stress conditions (2), which are of major importance in bacterial pathogenesis (1-3). For these reasons, the search for antiquorum sensing compounds (Anti-QS) offers a new perspective on the application of natural or pure compounds as therapeutic agents, which by inhibiting this mechanism of cell communication could be used to control bacterial diseases (4).
Nature offers a wide range of plants, algae and bacteria capable of inhibiting QS mechanisms. This fact has allowed the development of various studies aimed at finding new options for treatment of infections caused by pathogenic bacteria (5-9). Accordingly, QS systems are molecular targets with applications in various fields, including medicine, veterinary medicine, agriculture and aquaculture (3).
The search for antiquorum sensing compounds is possible by using various bioassays (10). Some of the most common bioassays are those performed with Chromobacterium violaceum CV026. This bacterium is a mutant of the ATCC 31532 strain, which is deficient in the production of N-hexanoyl homoserine lactone (C6-HSL), due to a mutation induced by insertion of a mini-Tn5 in the cviI gene, C. violaceum acyl-homoserine lactone (AHL) synthase, that controls the synthesis of violacein. Therefore, the quorum sensing mechanisms responsible for the formation of this pigment are activated after the exogenous addition of the autoinducer, AHL. This strain can be used as an indicator organism to detect short-chain molecules with structural similarities to C6-HSL, so that they could compete for the receptor and act as indicators of QS inhibition (10-15). Consequently, this approach has generated many reports plant extracts acting asinhibitors of quorum sensing. One of such extracts is Allium sativum, which potentiates the action of the antibiotic tobramycin against Pseudomonas aeruginosa, improving the outcome of pulmonary infection (16), Other plants from different families whose extracts exhibit QS-inhibiting properties are Conocarpus erectus (Combretaceae), Quercus virginiana (Fagaceae), Bucida buceras (Combretaceae), Callistemon viminalis (Myrtaceae), Tetrazygia bicolor (Melastomataceae), and Chamaecyce hypericifolia (Euphorbiaceae) (17). The aim of this study was to evaluate the antiquorum sensing activity of essential oils (EOs) of three species of the genus Piper growing in Colombia.
MATERIALS AND METHODS
The general methodology used in this study included field collection of plant material, isolation of EOs, and determination of their cell growth, and anti-QS activities as described below.
Bacterial strains and culture conditions.
The bacterial strain used in this study was Chromobacterium violaceum CV026, which is unable to produce violacein by itself. However, this biosynthesis can be induced by the presence of AHL, whose N-acyl side chains have a length of C4-C8. The C6-homoserine lactone (HHL) was dissolved in dimethylsulfoxide (DMSO), and it was used as autoinducer for violacein pigment production (16).
The bacterial strain, which was kept in Eppendorf tubes at -70ºC, was revived in Luria-Bertani agar (LB) (18) and incubated for 18 hours at 30°C. After this process, culture colonies were transferred to LB broth; cell density was read at a wavelength of 620 nm, and then adjusted to 0.5 in the Mc-Farland scale. This value is equivalent to an absorbance of 0.08-0.1 according to the National Committee for Clinical Laboratory Standards (NCCLS) (18-19).
Plant material and essential oil isolation.
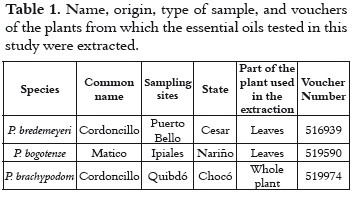
Three plant species of the genus Piper were used in the assays (Piper bogotense, Piper brachypodom and Piper bredemeyeri). Plant material was collected from several Colombian states (see table 1) according to information provided by ethnobotanical sources. Samples were properly transported to the laboratory of the Centro Nacional de Investigaciones para la Agroindustrialización de Especies Vegetales Aromáticas Medicinales Tropicales, CENIVAM (National Center for Research on Agro-industrialization of Tropical Medicinal Aromatic Plants), at Universidad Industrial de Santander (Industrial University of Santander), Bucaramanga (Colombia), where the essential oils were extracted. The material was classified systematically by botanist José Luis Fernández, from the Institute of Natural Sciences of Universidad Nacional de Colombia, UNAL (National University of Colombia). The specimens and their respective vouchers were deposited in the UNAL Herbarium. General information about the species, sampling sites, plant parts used in EO extraction, and voucher number are shown in table 1.
EOs from different parts of the plant (see table 1) were obtained using hydrodistillation, assisted by microwave radiation. For this hydrodistillation, the extraction equipment was placed next to a domestic microwave oven. A flask was connected to a condenser, and both of them were put into the oven. The flask contained the plant material (100 g) and water (2 L). The water was heated for 30 minutes and the resulting oil was isolated by decantation and dried using anhydrous sodium sulfate.
Preparation of essential oil dilutions.
Essential oils were initially dissolved in DMSO and then added to the culture medium to obtain concentrations of 0.01, 0.1, 1, 10, 50, 100, 200, 300, 400, 500, 750 and 1000 µg/mL. The maximum amount of DMSO used in the assays was 0.5%.
Measurement of cell growth
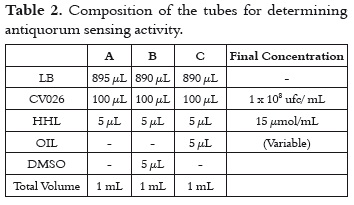
Cell growth inhibition was assessed following the broth dilution method, according to the recommendations of the NCCLS (19). For this, properly labeled tubes (duplicates) were used to deposit a bacterial culture and different essential oil concentrations (tube A), DMSO (tube B), and one containing neither oil nor DMSO (Tube C). Tube B shows the influence of DMSO on the bacterium, and tube C indicates bacterial behavior without the action of EOs and DMSO. Bacterial growth was identical in tubes B and C, thus suggesting that DMSO did not affect the bacterial culture.
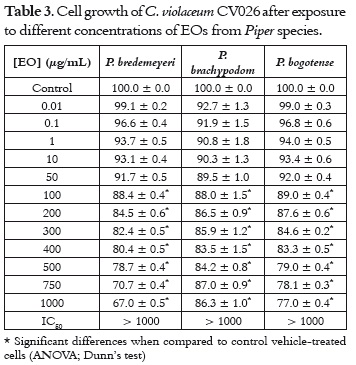
The strain was incubated for a period of 18 hours at 30°C, then adjusted to 0.5 according to the Mc-Farland scale, and 100 µL were placed in tubes containing LB broth and different EO dilutions. These tubes were incubated at 30°C for another 24 hours; then, absorbance was measured in a microplate reader at 620 nm (18). The absorbance data were normalized to vehicle-control, for which a cell growth rate of 100% was assumed. The number of colony-forming units (cfu) of CV026 was estimated on LB agar plates. Cells growing at a rate of 80% in comparison with control cells (cells exposed to 400 µg/mL P. bredemeyeri essential oil) were greater than 1.6 x 106 cfu/mL.
Measurement of antiquorum sensing activity.
Anti-QS activity measurements were performed as follows: a single colony of the biosensor strain was transferred to LB broth, and then allowed to grow at 30°C for 24 hours, and then the culture was adjusted to 0.5 on the corresponding Mc-Farland scale. 100 µL of this suspension were placed in separate tubes containing LB broth and HHL; LB broth, HHL and DMSO; and LB broth, HHL and different dilutions of essential oil (tubes A, B and C respectively, as shown in table 2). These tubes were incubated at a temperature of 30°C for 24 hours (18). All experiments were performed four times.
Quantifying violacein production.
After the incubation period, the tubes were vortexed to resuspend cells and biofilms; and 300 µL of this suspension were placed in 1.5 mL Eppendorf tubes. The cells were lysed with 300 µL of 10% sodium dodecyl sulfate, vortexed for 2 minutes, and then incubated at room temperature for 5 minutes. Violacein was extracted quantitatively adding 800 µL of a mixture of butanol/water 1:1, stirred for 5 seconds and then centrifuged at 13000 rpm for 5 minutes. Once centrifuged, the violacein, which was present at the upper layer, was carefully removed and its absorbance measured at 585 nm (11). Violacein concentration was normalized to the concentration obtained for the vehicle-control (100%).
Data analysis.
Results are presented as mean ± standard deviation (x ± SD). A probit analysis was used to calculate the 50% inhibitory concentration (IC50) for both cell growth and quorum sensing. This 50% inhibitory concentration was defined as the concentration of essential oil that leads to a 50% reduction of cell growth compared to vehicle-control, and the concentration of essential oil that leads to a 50% reduction of violacein production compared to the amount produced by C. violaceum when fully induced by C6-HSL, respectively. The differences between the means of the responses obtained for each tested concentration were evaluated by analysis of variance (ANOVA), after a logarithmic data transformation. Dunn's test was used to perform comparisons against the control group whenever significant differences were found between means. In all cases, the normal distribution and equality of standard deviations of the means were checked using the Kolmogorov-Smirnov and Bartlett tests, respectively. In the absence of normality, mean comparisons between more than two groups were performed by means of the Kruskal Wallys test. For all cases, the level of significance was set at p<0.05.
RESULTS AND DISCUSSION
Cell growth inhibition
The results obtained in this assay are presented in table 3. The EOs of P. bredemeyeri, P. bogotense and P. brachypodom have a minor effect on the growth of C. violaceum CV026. Although there is a clear doseresponse relationship, and a low reduction (≈ 10%) of cell growth at concentrations below 100 µg/mL, bacterial growth remained greater than 50% even at concentrations greater than 1000 µg/mL.
Antiquorum sensing activity.
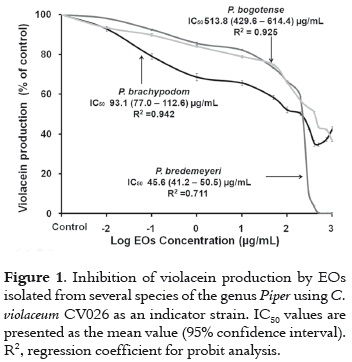
The anti-QS activity of the evaluated EOs is shown in figure 1. Anti-QS activity decreases in the following order: P. bredemeyeri> P. brachypodom> P. bogotense. For P. bredemeyeri and P. brachypodom IC50 was lower or similar to the concentration at which bacterial growth showed no significant differences compared to control cells. However, even for P. bogotense, the percentage of cell growth associated with the IC50 value is at least of 77%, which indicates that the anti-QS activity for these oils is basically independent of cell growth (7).
The chemical composition of the EOs evaluated in this study has been previously reported in the literature,andsomeof the major constituents (>10%) present in these species are: Sabinene/β-pinene and α-pinene for P. bredemeyeri; trans-β-caryophyllene and caryophyllene oxide for P. brachypodom; transsabinene hydrate and α-phellandrene for P. bogotense (20, 21). This presence clearly reveals the wide variety of chemical constituents that can be found in these species. Interestingly, the most active oil (P. bredemeyeri) has a high concentration rate (> 10%) of sabinene/β-pinene/α-pinene molecules, which are absent in P. brachypodom and P. bogotense, with the exception of α-pinene, which is present in P. bogotense at a concentration rate of 8.7%.
Piper species are traditionally used to treat diseases such as vaginitis and intestinal disorders; although, they have been shown to possess various pharmacological properties such as antiviral, antiinflammatory, antibacterial, and antifungal activities (22-24). In addition, EOs from this genus inhibit the growth of a large group of microorganisms that cause important human infections such as Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli; and the fungi Trichophyton mentagrophytes, Candida albicans, Aspergillus flavus and Aspergillus fumigatus (25).
There are several reports in the literature that reveal anti-QS activity in some essential oils, including rosemary, rose, geranium, lavender and clove (26, 27). These oils have constituents that are also present in the EOs evaluated in this study. For instance, α-pinene is present in rosemary oil; whereas linalool and α-humulene are present in rose oil, and limonene in lavender oil (28-30).
Different mechanisms have been proposed to explain the interference of quorum sensing- depending processes by natural products . Some of these mechanisms are the inhibition of signal molecule biosynthesis (1,5) or AHL signal reception (1,5), and the enzymatic inactivation and biodegradation of quorum sensing molecules (31). Although the mechanisms through which EOs inhibit HHLactivated QS systems are not known, the apolar nature and relative size of the components of these oils (similar to HHL) could represent an argument to suggest that these mixtures might be acting through a possible competitive inhibition with the HHL receptor. However, further studies are needed to examine this hypothesis.
The results presented in this paper indicate that the three evaluated EOs are able to inhibit quorum sensingbyusing the sensor strain C. violaceum CV026. This fact suggests that these oils are promising candidates for antibacterial drug development, and could provide an alternative to improve the response to antibiotics, through the intervention of a mechanism such as quorum sensing. Even though, there is still no evidence associated with bacterial resistance (5) for such mechanism.
CONCLUSION
The essential oils of Piper bredemeyeri, Piper bogotense and Piper brachypodom are capable of inhibiting quorum sensing on C. violaceum CV026, and these effects occur at concentrations that induce low disruption of cell growth on the sensor strain. Accordingly, these oils are good candidates for the development of anti-QS molecules with potential applications for the control of bacterial diseases mediated by quorum sensing.
ACKNOWLEDGMENTS
The authors thank Colciencias-CENIVAM-Universidad de Cartagena (Project RC-432-2004), Vice-Rectory for research of the Universidad de Cartagena (Research Group Support Program, 2009-2010), Cartagena, Colombia; Dr. Robert McLean, Department of Biology, Texas State University – San Marcos for donating the bacterial strain C. violaceum CV026; and Ana Carolina Barreto and Rosa Baldiris Ávila for their invaluable professional support.
REFERENCES
1. Vattem DA, Mihalik K, Crixell SH, McLean R. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia. 2007 Jun; 78 (4): 302-310. [ Links ]
2. Gobbetti M, De Angelis M, Di Cagno R, Minervini F, Limitone A. Cell-cell communication in food related bacteria. Int J Food Microbiol. 2007 Nov 30; 120 (1-2): 34-45. [ Links ]
3. Bai F, Han Y, Chen J, Zhang XH. Disruption of quorum sensing in Vibrio harveyi by the AiiA protein of Bacillus thuringiensis. Aquaculture. 2008 Jan 31; 274 (1): 36-40. [ Links ]
4. Domingo D, López M. Plantas con acción antimicrobiana. Rev Esp Quimioter. 2003 Dec; 16 (4): 385-393. [ Links ]
5. Hentzer M, Givskov M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest. 2003 Nov 1; 112 (9): 1300-1307. [ Links ]
6. Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999 Jun; 86 (6): 985-990. [ Links ]
7. Rasmussen TB, Givskov M. Quorum-sensing inhibitors as antipathogenic drugs. Int J Med Microbiol. 2006 Apr 6; 296 (2-3): 149-161. [ Links ]
8. Acosta M, González M, Araque M, Velazco E, Khouri, N, Rojas L, et al. Composición química de los aceites esenciales de Ocimum basilicum L. var basilicum, O. basilicum L. var purpurenscens, O. gratissimum L., y O. tenuiflorum L, y su efecto antimicrobiano sobre bacterias multirresistentes de origen nosocomial. Rev Fac Farm. 2003 Jan-Jun; 45 (1): 19-24. [ Links ]
9. Rasmussen TB, Skindersoe ME, Bjarnsholt T, Phipps RK, Christensen KB, Jensen PO, et al. Identity and effects of quorumsensing inhibitors produced by Penicillium species. Microbiology. 2005 May; 151 (Pt 5): 1325-1340. [ Links ]
10. McLean RJ, Pierson LS, Fuqua C. A simple screening protocol for the identification of quorum signal antagonists. J Microbiol Methods. 2004 Sep; 58 (3): 351-360. [ Links ]
11. Blosser RS, Gray KM. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J Microbiol Methods. 2000 Mar; 40 (1): 47-55. [ Links ]
12. McClean KH, Wilson MK, Fish L, Taylor A, Chhabra SR, Camara M, et al. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997 Dec; 143 (Pt 12): 3703-3711. [ Links ]
13. Gomes C. Regulação Gênica da Biossíntese de Violaceína e Quorum sensing em Chromobacterium violaceum [Tesis doctoral]. Florianópolis SC-Brasil: Universidade Federal de Santa Catarina. 2005. 30-34 p. [ Links ]
14. Zinder-yosovich K, Sudakevitz D, Imberty A, Garber NC, Gilboa-Garber N. Production and properties of the native Chromobacterium violaceum fucose-binding lectin (CV-IIL) compared to homologus lectins of Pseudomonas aeruginosa (PAIIL) and Ralstonia solanacearum (RS-IIL). Microbiology. 2006 Feb; 152 (Pt 2): 457-463. [ Links ]
15. Williams P. Quorum sensing, communication and crosskingdom signalling in the bacterial world. Microbiology. 2007 Dec; 153 (Pt 12): 3923-3938. [ Links ]
16. Bjarnsholt T, Jensen PO, Rasmussen TB, Christophersen L, Calum H, Hentzer M, et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology. 2005 Dec; 151 (Pt 12): 3873-3880. [ Links ]
17. Adonizio AL, Downum K, Bennett BC, Mathee K. Anti-quorum sensing activity of medicinal plants in southern Florida. J Ethnopharmacol. 2006 May 24; 105 (3): 427-435. [ Links ]
18. Choo JH, Rukayadi Y, Hwang JK. Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiology. 2006 Jun; 42 (6): 637-641. [ Links ]
19. NCCLS (National Committee for Clinical and Laboratory Standards Institute). Métodos de dilución para determinar la sensibilidad a los antimicrobianos de bacterias que crecen en condiciones aeróbicas; Norma Aprobada, Séptima Edición. 2006; 26 (2): 11-12; 16-19 p. [ Links ]
20. Olivero J, Guette J, Stashenko E. Acute toxicity against Artemia franciscana of essential oils isolated from plants of the genus Lippia and Piper collected in Colombia. BLACPMA. 2009 Sep; 8 (5): 419-427. [ Links ]
21. Castañeda M, Muñoz A, Martínez J, Stanshenko E. Estudio de la composición química y la actividad biológica de los aceites esenciales de diez plantas aromáticas Colombianas. Scientia et Technica. 2007 Abr; 13 (33): 165-166. [ Links ]
22. Stohr JR, Xiao PG, Bauer R. Constituents of Chinese Piper species and their inhibitory activity on prostaglandin and leukotriene biosynthesis in vitro. J Ethnopharmacol. 2001 May; 75 (2-3): 133-139. [ Links ]
23. Dos santos PR, de Limas Moreira D, Guimaraes EF, Kaplan MA. Essential oil analysis of 10 Piperaceae species from the Brazilian Atlantic Forest. Phytochemistry. 2001 Oct; 58 (4): 547-551. [ Links ]
24. Pino N, Yurgaki T, Cuesta J. Aspectos botánicos y química preliminar de seis especies del género Piper usadas como medicinales en el municipio de Quibdó-Chocó. Rev Inst Univ Tecnológica Chocó. 2005 Jul-Dic; 23 (2): 20-25. [ Links ]
25. Mesa A, Montiel J, Martínez C, Zapata B, Pino N, Bueno, et al. Actividad in vitro anti-candida y anti-aspergillus de aceites esenciales de plantas de la familia Piperaceae. Scientia et Technica. 2007 Abr; 13 (33): 247-249. [ Links ]
26. Szabó MA, Varga GZ, Hohmann J, Schelz Z, Szegedi E, Amaral J, et al. Inhibition of quorum-sensing signals by essential oils. Phytother Res. 2010 May; 24 (5): 782-786. [ Links ]
27. Khan MS, Zahin M, Hasan S, Husain FM, Ahmad I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett Appl Microbiol. 2009 Sep; 49 (3): 354-360. [ Links ]
28. Santoyo S, Cavero S, Jaime L, Ibañez E, Señoráns FJ, Reglero G. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. essential oil obtained via supercritical fluid extraction. J Food Protect. 2005 Apr; 68 (4): 790-795. [ Links ]
29. Umezu T, Nagano K, Ito H, Kosakai K, Sakaniwa M, Morita M. Anticonflict effects of lavender oil and identification of its active constituents. Pharmacol Biochem Behav. 2006 Dec; 85 (4): 713-721. [ Links ]
30. Wom MM, Cha EJ, Yoon OK, Kim NS, Kim K, Lee DS. Use of headspace mulberry paper bag micro solid phase extraction for characterization of volatile aromas of essential oils from Bulgarian rose and Provence lavender. Anal Chim Acta. 2009 Jan 5; 631 (1): 54-61. [ Links ]
31. Defoirdt T, Boon N, Dossier P, Verstraete W. Disruption of bacterial quorum sensing: an unexplored strategy to fight infections in aquaculture. Aquaculture. 2004 Oct 27; 240 (1-4): 69-88. [ Links ]