Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.18 no.2 Medellín May/Aug. 2011
BIOTECHNOLOGY
MICROBIAL BIOTRANSFORMATION OF (R)-(+)- LIMONENE BY Penicillium digitatum DSM 62840 FOR PRODUCING (R)-(+)-TERPINEOL
BIOTRANSFORMACIÓN MICROBIANA DE (R)-(+)-LIMONENO POR Penicillium digitatum DSM 62840 PARA LA PRODUCCIÓN DE (R)-(+)-TERPINEOL
Gloria A. PRIETO S.1; Janeth A. PEREA V.2; Claudia C. ORTIZ L.3
1 Escuela de Química, Facultad de Ciencias Básicas, Universidad Pedagógica y Tecnológica de Colombia, Tunja-Colombia.
2 Centro de Investigaciones en Ciencia y Tecnología de Alimentos –CICTA, Escuela de Química, Universidad Industrial de Santander, Km 2 ruta Guatyguara. Piedecuesta, Colombia. aperea@uis.edu.co.
3 Escuela de Bacteriología y Laboratorio Clínico, Universidad Industrial de Santander. Calle 9a - Carrera 27. Bucaramanga, Colombia.
Received: 20 September 2010; Accepted: 18 July 2011
ABSTRACT
Microbial biotransformation is a relevant strategy to obtain high added value natural compounds under controlled environmentally friendly conditions. In this research work, the biotransformation of (R)-(+)- limonene using Penicillium digitatum DSM 62840 was evaluated. The study variables were the following: culture medium, pH, microorganism growth phase, substrate concentration, and inducing effect of the substrate. The results showed that a concentration of 14.7 mM limonene in the medium named malt yeast broth at a pH of 3.5, inoculated with induced spores at the early stage of the exponential growth, produces a high specificity and the highest concentration (1864 mg/L) of (R)-(+)-α-terpineol. The product obtained has a considerable potential industrial application.
Keywords: Bioconversion, biotransformation, monoterpenes, fungi, α-terpineol.
ABSTRACT
La biotransformación microbiana es una estrategia relevante para obtener compuestos naturales de alto valor agregado a través de procesos amigables con el medio ambiente. En este estudio se evaluó la biotransformación de (R)-(+)-limoneno utilizando Penicillium digitatum DSM 62840. Las variables estudiadas fueron: medio de biotransformación, pH, fase de crecimiento del microorganismo, concentración del sustrato y efecto inductor del sustrato. Los resultados mostraron que en medio caldo extracto de malta y levadura a pH 3,5 inoculado con esporas inducidas y crecidas en el inicio de la fase exponencial, con una concentración de limoneno de 14,7 mM, el sustrato se transformó de manera específica en (R)-(+)- α-terpineol. En estas condiciones se alcanzó la máxima concentración, 1864 mg/L. El producto obtenido tiene alto potencial de aplicación en diferentes industrias.
Palabras clave: bioconversión, biotransformación, monoterpenos, hongo, α-terpineol.
INTRODUCTION
Biocatalytic processes under controlled conditions are being more frequently used in processes of chemical synthesis towards a sustainable development, green chemistry and the implementation of environmentally benign processes. These processes offer more advantages compared to conventional catalysts such as mild reaction conditions, high catalytic activities, and a higher regio/stereo selectivity (1-3). These characteristics become relevant in the synthesis of pharmaceuticals and in the process of flavoring products; therefore, this fact allows classifying them as ''natural substances'' according to the established official criteria in USA and Europe (4). Industrial biotransformations are frequently used to produce different types of chemical compounds and natural substances from both cheap and readily available substrates (5).
(R)-(+)-limonene is the most abundant monoterpene in nature. It represents over 90% of the essential oil extracted from citrus fruit peels (6) and it is used as a substrate for the synthesis of terpene derivatives that have a significant importance in the production of food, as well as in the pharmaceutical and perfumery industry (6-8). Limonene can be biotransformed into oxygenated monoterpenes such as α-terpineol, which is a monoterpenoid that has a significantly higher added value than limonene (100 times more, USD $78/0.5 lb), and an market of 13,000 tons commercialized per year. Moreover, α-terpineolis is considered to be a safe additive (GRAS 3045), because it has a characteristic aroma of lavender, which is commonly used as fragrance in the industry of perfumes, fragrances, cosmetics and toiletries (9, 10). It is also used in the pharmaceutical industry as an antifungal and disinfectant product (9, 11), and in the food industry as a preservative due to its antimicrobial and antioxidant properties (12-14).
Limonene can be transformed into α-terpineol using strains from different fungi, including Fusariumo xysporum 152B (15-17), Cladosporium (18), Pleurotus sapidus (19), Aspergillus niger (ATCC 16404, ATCC 9642 and ATCC 1004 strains) (20) and Penicillium spp. isolated from orange peel (21, 22), as well as strains from plant cells (23-27). α-terpineol can also be produced from bacteria such as Pseudomonas gladioli (28, 29), Escherichia coli, (30) and Sphingobium spp. (31). In all cases, the process is highly enantiospecific (15, 18, 23, 24, 31), but the product concentration depended on the reaction conditions.
The physicochemical α-terpineol properties that were obtained by biotransformation of limonene were determined to confirm its identity: specific gravity, refractive index, solubility in ethyl alcohol (70%), and acid number. The results were 0.932, 1484, 1, and up to 10 mL and 0.02 mg KOH/g oil, respectively (22).
In this study the biotransformation of (R)- (+)-limonene by P. digitatum DSM 62840 was evaluated. The influence of the culture medium, pH, microorganism growth phase, substrate concentration and inducing effect of the substrate were considered. The microbial growth kinetics of Penicillium digitatum DSM 62840 in a solid medium, as well as the antifungal activity of the substrate had been previously analyzed.
MATERIALS AND METHODS
Microorganism, culture media and reagents
Penicillium digitatum DSM 62840 was obtained from the German Collection of Microorganisms and Cell Cultures –DSMZ– (Braunschweig, Germany). The culture media PDA (potato dextrose agar), MEA (malt extract agar) and the raw materials required to prepare the YGA complex medium (yeast extract 3.0 g /L, malt extract 20 g/L, glucose 20 g/L, bacteriological peptone 1.0 g/L, and agar 20 g/L), the YMPG (yeast extract 5.0 g/L, malt extract 10 g/L, glucose 10 g/L, and bacteriological peptone 5.0 g/L), the MYB (yeast extract 3.0 g/L, malt extract 20 g/L, glucose 10 g/L, and bacteriological peptone 10 g/L) and the YG (yeast extract 3.0 g/L, malt extract 20 g/L, glucose 20 g/L, and bacteriological peptone 1.0 g/L) were purchased from OXOID® (Hampshire, England). (R)-(+)-limonene (98%) and α-terpineol (98%) were purchased from Merck (Darmstadt, Germany).
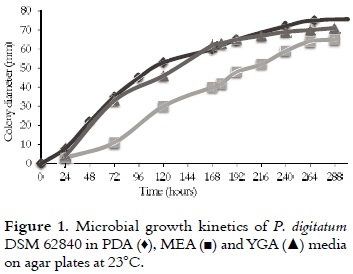
Microbial growth kinetics of Penicillium digitatum DSM 62840 in solid medium
Penicillium digitatum DSM 62840 was grown in PDA, MEA and YGA media at 23°C for 10 days. In all cases, the colony diameter was measured over time in order to determine the microbial growth kinetics of the microorganism. The radial growth rate (RGR, mm/h) was determined according to the method described by Trinci, 1969 (32) and Pirt, 1975 (33). The best growth medium for microbial cultures was selected according to the RGR values, and it was later used for the maintenance of the strain. All tests were performed in triplicate.
Substrate antifungal activity evaluation
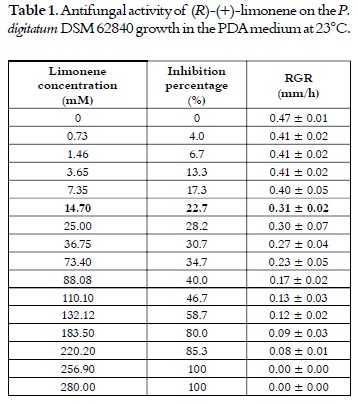
Different concentrations (0 - 280 mM) of (R)- (+)-limonene were added in the PDA medium before gelation. In all cases, the dispersion of (R)-(+)- limonene was carried out for agitation. Subsequently, the media were inoculated with 20 μL of spore suspension –SS– (1 x 107 spores/mL). Microbial growth (at 23°C) was monitored by measuring the change in diameter of colonies after 8 days (34, 35). The percentages of inhibition were calculated using equation 1, where Dc is the witness fungal colony average diameter, and De is the average diameter of the test. The results were used to produce the inhibition curves. The smallest concentration of (R)-(+)-limonene, which results in a zone of growth inhibition of P. digitatum DSM 62840, is called minimum inhibitory concentration (MIC); and the concentration that inhibited 100% of growth is called lethal concentration (CL).
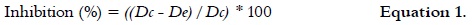
Preparation of spore suspension
The spores obtained in the previously selected solid medium were resuspended in 10 mL of saline solution (0.85% NaCl and 0.1% Tween® 80), and a recount of number (N) of cells per mL was done in a Neubauer chamber. The initial concentration of spores in the biotransformation medium was 1 x 107 spores/mL.
Microbial growth kinetics of Penicillium digitatum DSM 62840 in liquid medium
Penicillium digitatum DSM 62840 was growth in 22 mL vials fitted with teflon stoppers, containing 5 mL of sterile YMPG, MYB or YG liquid medium, at 27°C and an agitation of 150 rpm using an orbital shaker (Heidolph®, Vibramax 100, Schwabach, Germany). The medium was inoculated with 50 μL of SS. The biomass concentration was determined through the dry weight cell method, taking samples every 24 h for 15 days (36).
General process of limonene biotransformation at laboratory scale
The bioassays were carried out in 22 mL vials fitted with teflon stoppers, containing 5 mL of sterile liquid medium. The culture medium was inoculated with 50 μL of SS and pre-incubated at 27°C for 72 h with an agitation of 150 rpm, using an orbital shaker. After pre-incubation, pure (R)-(+)- limonene was added at specific concentrations and conditions (see the results section) established for each experiment. The microbial biotransformation was monitored throughout the whole experiment. At the same time, two controls, biomass blank (spores suspended in the reaction medium without substrate) and blank substrate (reaction medium and substrate without suspension of spores), were prepared. The reaction products and remaining substrate were extracted and analyzed through GC-MS. Bioconversion (mg α-terpineol/mg, initial substrate*100) was evaluated in all cases.
Effect of type of culture medium
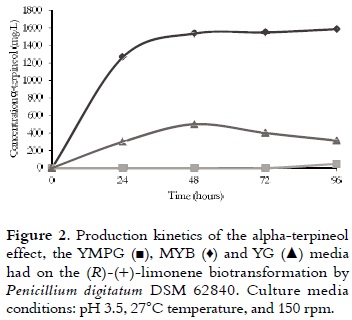
Biotransformation experiments were carried out in liquid media, using YMPG, YG or MYB dissolved in a 0.1 M citrate-phosphate buffer (pH 3.5) and under the environmental conditions specified above. (R)-(+)-limonene was added at a final concentration of 14.7 mM. Kinetics of microbial transformation was monitored at 0, 24, 48, 72 and 96 h after adding the substrate. The culture medium with the highest bioconversion values was selected for evaluating additional biotransformation parameters.
Effect of pH
The effect of pH on limonene biotransformation was studied by dissolving MYB culture medium in a 0.1 M citrate-phosphate buffer at the following pH values: 3.0, 3.5, 4.5 and 6.0. The bioassays were carried out in 22 mL vials fitted with teflon stoppers, containing 5 mL of sterile liquid medium. Culture media were inoculated with 50 μL of SS and pre-incubated at 27°C for 72 h, with an agitation of 150 rpm using an orbital shaker. (R)-(+)-limonene was added at a final concentration of 14.7 mM. The microbial transformation was monitored at 0 and 48 h after adding the substrate. The culture medium with the highest specificity and bioconversion values was selected for evaluating additional biotransformation parameters.
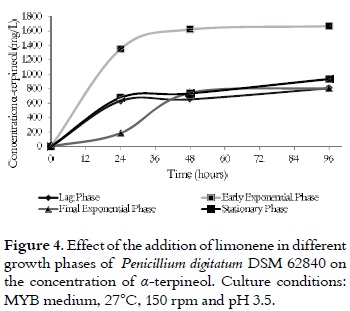
Evaluation of the microorganism growth phases on limonene biotransformation
The bioassays were carried out in 22 mL vials fitted with teflon stoppers, containing 5 mL of sterile liquid MYB medium (pH 3.5). Culture media were inoculated with 50 μL of SS and pre-incubated at 27°C for 24, 72, 120, 168 and 216 h, according to the lag, early exponential, media exponential, final exponential and stationary phases respectively, with an agitation of 150 rpm using an orbital shaker. Once P. digitatum reached the corresponding phase, (R)-(+)-limonene was added to the culture MYB medium (pH 3.5) at a final concentration of 14.7 mM. The microbial biotransformation kinetics for each biotransformation was monitored at 0, 24, 48 and 96 h after the reaction. The growth phase with the highest bioconversion was selected for evaluating further parameters.
Effect of substrate concentration
Different concentrations of (R)-(+)-limonene (5 - 100 mM) were used. The bioassays were carried out in 22 mL vials fitted with teflon stoppers, containing 5 mL of sterile liquid MYB medium (pH 3.5). Culture media were inoculated with 50 μL of SS and pre-incubated at 27°C for 72 h (early exponential phase) with an agitation of 150 rpm. (R)-(+)-limonene was added at a final concentration of 5 - 100 mM. The microbial transformation was monitored at 0 and 48 h after adding of the substrate. The optimal substrate concentration was selected according to both higher specificity and higher bioconversion.
Inducing effect of (R)-(+)-limonene
In order to determine the possible inducible effect of substrate, biotransformation experiments were carried out by inoculating the MYB medium, at a pH of 3.5, with 50 μL of SS, which was previously obtained from a fungal culture grown in the presence of the inducer (1.47 mM (R )-(+)- limonene). The biotransformations were carried out for 72 h at 27°C and 150 rpm, with 14.7 mM (R)-(+) limonene in the culture medium, and they were monitored at 0, 8, 24 and 48 h.
Extraction, identification and quantification of (R)-(+)- limonene and (R)-(+)-α-terpineol during the limonene biotransformation
The products and remaining substrates in the biotransformation reactions were extracted two times with ethyl acetate (2 x 2.5 mL), followed by centrifugation at 4000 rpm for 5 min. The organic phase was collected and dried with anhydrous Na2SO4, and then concentrated with a N2 stream. Subsequently, 3 μL of n-tetradecane (internal standard) were added and diluted to 1 mL. The substrate and oxygenated products were identified and quantified through a gas chromatography coupled with a mass spectrometry (GC-MS), using an Agilent® chromatograph model 6890N (Palo Alto, CA, USA) coupled to an Agilent® Technologies 5975 C mass selective detector with an electron impact ionization detector system (70 eV) and a quadrupole mass analyzer, operated in full scan mode from 40-400 Dalton (m/z). A split ratio of 1:13 and a HP 7683 Series automatic injector were used for this research. Also, a DB-WAX capillary column (60m long x 0.25mm inner diameter x 0.25 μm film thickness) was used. Temperature was programmed at 45°C and maintained for 10 min; then, temperature was raised at a heating rate of 3°C/min until reaching 220°C, maintaining this final temperature for 30 min. The identification of compounds was performed comparing the mass spectra of the samples with the following compound spectra library data from the ADAMS database: NSB 75K, 138K NIST 05 and WILEY, available in the G1701BA HP Enhanced Chemstation data systems. The concentrations of limonene and α-terpineol in each extract were quantified from individual calibration curves, using peak area ratios (analyte/i.S) vs. amount ratios (analyte/i.S) from the standard authentic samples.
The product recovery efficiency from ethyl acetate liquid-liquid extraction was previously determined in five separate extractions with known amounts of authentic α-terpineol and limonene in a fresh growth medium and spent cell broth. The standards were extracted, and the amounts of extracted α-terpineol and limonene were previously determined as it was described. Each test was conducted in triplicate, and the average percentage recovery rates were calculated.
Statical analysis
Antifungal activity assays were performed in triplicate. Biotransformation experiments were performed twice and each test was carried out in duplicate for a total of four samples for experiment. The remaining substrate concentrations and biotransformation products were expressed as the mean value ± standard deviation.
RESULTS AND DISCUSSION
Firstly, the microorganism growth was evaluated in different culture media (PDA, MEA and YGA). P. digitatum DSM 62840 grew in PDA and YGA in a similar way and at a higher growth rate than in the MEA medium, as it is shown in figure 1. The highest RGR (0.35 ± 0.01 mm/h) was obtained in the PDA medium. Therefore, PDA was selected for the periodic maintenance of P. digitatum DSM 62840, and for obtaining the spore solutions used in further experiments.
Evaluation of the substrate antifungal activity
To determine the antifungal activity of (R)- (+)-limonene on the growth of P. digitatum, the substrate concentration was varied in the range of 0 - 280 mM in the PDA medium. The addition of limonene to the culture medium adversely affected the P. digitatum growth as it is shown in table 1. Based on these results, a concentration of 0.73 mM was established as the minimum inhibitory concentration (MIC); while the concentrations above 257 mM were considered to be lethal concentrations (LC). This effect can be attributed to the toxicity of (R)-(+)-limonene. That is why several authors (8, 16, 18, 20, 23) have established that terpenes with n-octanol/water partition coefficients (log PO/W) between 1 and 5, (R)-(+)-limonene have a log PO/W of 4.8, which causes the loss of the specific permeability and integrity of the cell membrane (19, 21, 23).
For the biotransformation assays a 14.7 mM (R)- (+)-limonene substrate concentration was selected, value at which there is an abrupt change in RGR and an inhibition of 22.7%.
Evaluation of the (R)-(+)-limonene biotransformation process
Effect of the type of culture medium
The culture media that was used in this study contained similar nutrient components with different concentrations. With MYB and YG media, limonene was biotransformed only in (R)-(+)-α-terpineol. However, at 48 h of reaction, the concentration of (R)-(+)-α-terpineol was approximately 3 times higher (1585 ± 19.59 mg/L) in MYB than in YG, as it is shown in figure 2. In the YMPG media, products of limonene biotransformation were detected after 96 h of reaction, from which only trans-carveol was formed. The above mentioned results showed that the culture medium can affect both specificity and product concentration. Tan et al., 1998 (23) found a content of 220 mg of α-terpineol/g of dry cell after 48 h of reaction using limonene as substrate and Penicillium digitatum NRRL 1202 as the fungi strain; while, in this study, the specific content was 4 times higher (880.6 ± 2.64 mg of α-terpineol/g of dry cell). Additionally, the low biotransformation of the YG media was possibly due to a catabolic repressionlike mechanism by a high glucose concentration, which led to the decrease in the use of limonene as a source of carbon and energy. Similar effects have been reported in other studies (16, 22). According to these results, the MYB medium was selected for further studies.
Effect of pH
The biotransformation ability of P. digitatum was evaluated at different pH levels on the MYB medium. P. digitatum cells showed specificity toward the (R)-(+)-α-terpineol production at pH 3.0 and 3.5; while at pH levels over 4.5, this specificity decreased due to the fact that other products were obtained but terpineol was the major product in all cases, as it is shown in table 2. pH levels greater than 3.5 produced an intramolecular rearrangement that promoted the formation of the other oxygenated compounds of limonene (21) by means of hydroxylation reactions in different carbons of limonene, isomerization reaction, oxidation reaction and break cycles, as it is shown in figure 3. Moreover, the highest production of (R)-(+)-a- terpineol (1537 ± 34.95mg/L) was obtained at pH 3.5. For this reason, a pH value of 3.5 was selected to continue the studies.
At the pH levels of 4.5 and 6.0, the oxygenated derivatives linalool and isomers cis/trans-p-menth- 2,8-dien-1-ol are formed as it is shown in figure 3. These compounds have not been previously reported by limonene biotransformation. It is possible that the slightly acid pH, lead to a novel metabolic pathway for limonene by P. digitatum DSM 62840 cells. Linalool may be formed through intermediary a-terpinyl cation, as it naturally occurs in plants (37). The bioconversion of (R)-(+)- limonene by P. digitatum to α-terpineol implicated an intermediate (limonene-8,9-epoxide), which is formed via epoxidation for attack at the 8, 9-double bond, followed by a reductive cleavage occurring in α-terpineol (22, 23, 27). Epoxidation reactions are often catalysed by cytochrome P450-dependent mono oxygenases (15, 23).
Evaluation of the effect of the growth phases
In these experiments, limonene was added in the culture medium in different growth phases of the microorganism; its biotransformation was monitored every 24 h for 96 h. In all cases, Penicilliun digitatum cells produced (R)-(+)-α-terpineol. However, the highest production rate (1667 ± 49.70 mg/L) was obtained when limonene was added in the early exponential growth phase (72 h). (R)-(+)-α-terpineol production decreased between 50% and 60% in the culture medium when limonene was added in different growth phases. Moreover, in all cases, (R)-(+)-limonene biotransformation was quickly produced in the first 24 h. After 48 h of reaction, the biotransformation rate was constant. Figure 4 summarizes these results.
The growth phase effect on the limonene biotransformation has been previously evaluated by Tan et al., 1998 (23) using P. digitatum NRRL 1202 in a MYB medium at pH 4.5. The authors found that limonene biotransformation only occurs between early phase and the half of the exponential phase. However, there are no reports of obtained concentrations of (R)-(+)-α-terpineol. For this reason, it was not possible to compare these data with our results.
Effect of substrate concentration
Generally, high concentrations of organic solvents are toxic for biological systems (20, 21). To determine the optimum concentration of limonene for maximum bioconversion, product formation was measured over a range of 10 to 100 mM. The differences on limonene concentration affected both the specificity and the bioconversion of limonene by P. digitatum. In this study, concentrations between 10 and 15 mM favor α-terpineol production; while at higher concentrations (>30 mM), specificity decreased forming derived oxygenated such as cis/transcarveol, carvone, 1,2 - limonene diol, cis p-menth-2,8-dien-1-ol, phenyl ethanol (peaks 5, 6, 7, 8, 9 and 12, respectively), β-pinene, sabinene and mircene (peaks 1, 2 and 3), and two non-identified (peaks 11 and 14), as it is shown in figure 5.
The bioconversion percentages varied according to the substrate concentration, reaching the highest bioconversion percentage (75.21 ± 1.25%) at a limonene concentration of 15 mM (table 3). As substrate concentrations increased from 30 to 100 mM, a reduction in bioconversion percentages was observed. With Penicillium digitatum DSM 62840, optimal concentrations of substrate for a limonene biotransformation of 9.5 mM (26) have been published; while for other fungi, best results have been obtained with a limonene concentration of 62 mM (23) . The P. digitatum system appears to be less sensitive to this solvent than other reported strains (18, 28).
Inducible effect of (R)-(+)-limonene on the biotransformation capacity
The prior addition of limonene to induce biocatalytical machinery of fungal cells increased α-terpineol production in 14.58% according to the control at 48 h of reaction, as it is shown in table 4. Results suggest that the enzymes responsible for limonene oxyfuncionalization are inducible. The substrate induction of hydroxylation systems has been reported for several fungi strains, such as P. digitatum NRRL 1202 (23), Cladosporium (18) and P. gladioli (28). It is probable that the inducible system for the bioconversion of limonene by P. digitatum is a cytochromo P-450-dependent mono-oxigenase system, similar to the hydroxylation systems of other fungi and yeast (23).
Finally, based on previous results, it has been established that the specificity towards terpineol production and substrate bioconversion depended on culture medium, pH, microorganism growth phase, substrate concentration, and substrate induction. In this study, a concentration of α-terpineol and a specific activity of bioconversion four times higher than in other studies carried out with the same fungal strain (21, 26) were found. Also, bioconversion was higher compared with other fungi (15, 16, 23, 25). Furthermore, in this research, limonene was added in pure form, contrary to other studies in which it was added along with the co-solvent (20, 23).
CONCLUSIONS
(R)-(+)-limonene biocatalytic transformation by Penicillium digitatum DSM 62840 was highly dependent on the biotransformation medium, pH, growth phase of the fungus, and substrate concentration. In this work, the process forming (R)-(+)-α-terpineol was very specific. The highest conversion percentage (89.79%) was obtained using 15 mM of limonene in MYB medium at a pH of 3.5, inoculated with inducible spores at the early stage of the exponential growth, and with a total reaction time of 48 h. The production of α-terpineol by this and similar process can constitute a simple and efficient alternative for the production of flavor and fragrance agents, as well as antimicrobials and antioxidants.
ACKNOWLEDGEMENTS
We would like to thank Colciencias, the National Center for Research on Agro-industrialization of Aromatic and Medicinal Plant Tropical –CENIVAM– for financing this research (by means of the RC CENIVAM-COLCIENCIAS 432-2004 agreement). We would also like to thank the Pedagogical and Technological University of Colombia –UPTC, for a PhD fellowship for Gloria Astrid Prieto. Finally, we would like to thank the Industrial University of Santander, Colombia, for its technical support.
REFERENCES
1. Bommarius AS, Riebel BR, Bettina R. Biocatalysis. Fundamentals and applications. 1a Ed. Weinheim, Germany: Wiley-VCH; 2004. p. 23-72. [ Links ]
2. De Carvalho CCR, Da Fonseca MMR. Biotransformation of terpenes. Biotechnol Adv. 2006 Mar-Apr; 24 (2): 134-142. [ Links ]
3. Held M, Schmid A, van Beilen JB, Witholt B. Biocatalysis. Biological systems for the production of chemicals. Pure App Chem. 2000; 72 (7): 1337-1343. [ Links ]
4. Serra S, Fuganti C, Brenna E. Biocatalytic preparation of natural f lavours and fragrances. Trends Biotechnol. 2005 Apr 2; 23 (4): 193-198. [ Links ]
5. Thomas SM, Di Cosimo R, Nagarajan V. Biocatalysis: Applications and potentials for the chemical industry. Trends Biotechnol. 2002 Jun; 20 (6): 238-242. [ Links ]
6. Schrader J, Etschmann MMW, Sell D, Hilmer JM, Rabenhorst J. Applied biocatalysis for the biosynthesis of natural f lavor compounds-current industrial processes and future prospects. Biotechnol Lett. 2004; 26: 463-472. [ Links ]
7. Guenter M. The flavour and fragrance industry-past, present, and future. Berlin, Germany: Springer Verlag; 2007. p. 14-56. [ Links ]
8. Ohloff G. Scent and fragrances. The fascination of odors and their chemical perspectives. Berlin, Germany: Springer Verlag; 1994. 238 p. [ Links ]
9. Fisher K, Phillips C. Potential antimicrobial uses of essential oils in food: is citrus the answer?. Trends Food Sci Tech. 2008 Mar 13; 19 (3): 156-164. [ Links ]
10. Bathia SP, Letizia CS, Api AM. Fragrance material review on alpha-terpineol. Food Chem Toxicol. 2008 Jul 1; 46 (11): S280- S285. [ Links ]
11. Jun M, Jeong WS. Health promoting properties of natural flavours substances. Food SciBiotechnol. 2006 Jun; 15 (3): 329-338. [ Links ]
12. Schelz Z, Molnar J, Hohmann J. Antimicrobial and antiplasmid activities of essentials oils. Fitoterapia. 2006 May 11; 77 (4): 279- 285. [ Links ]
13. Maróstica MR, Rocha TAA, Franchi GC, Nowill A, Pastore GM, Hyslop S. Antioxidant potential of aroma compounds obtained by limonene biotransformation of orange essential oil. Food Chem. 2009 Feb 5; 116 (1): 8-12. [ Links ]
14. Bicas JL, Neri-Numa IA, Ruiz ALTG, De Carvalho JE, Pastore GM. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem Tox. 2011 Apr 19; 49 (7): 1610-1615. [ Links ]
15. Maróstica MR Jr, Pastore GM. Production of (R)-(+)-a-terpineol by the biotransformation of limonene from orange essential oil, using cassava waste water as medium. Food Sci. 2005 Mar 20; 101 (1): 345-350. [ Links ]
16. Bicas JL, Cavalcante FF, Wagner R, Godoy HT, Pastore GM. Optimization of (R)-(+)-a-terpineol production by the biotransformation of (R)-(+)-limonene. J Ind Microbiol Biot. 2008 Jun 17; 35 (9): 1061-1070. [ Links ]
17. Bicas JL, Pereira C, Néri IA, Pastore GM. Integrated process for co-production of alkaline lipase and R-(+)-a-terpineol by Fusarium oxysporum. Food Chem. 2009 Oct 20; 120 (2): 452-456. [ Links ]
18. Kraidman G, Mukherjee BB, Hill ID. Conversion of D-limonene into an optically active isomer of a-terpienol by a Cladosporium species. Bacteriol Proc. 1969; 69: 63. [ Links ]
19. Onken J, Berger RG. Effets of R-(+)-limonene on submerged cultures of the terpene transforming basidiomycete Pleurotus sapidus. J Biotechnol. 1999 Apr 15; 69 (2-3): 163-168. [ Links ]
20. Rottava I, Toniazzo G, Cortina PF, Martello E, Grando CE, Lerin LA, et al. Screening of microorganisms for bioconversion of (-)-ß-pinene and R-(+)-limonene to a-terpineol. LWT - Food Sci Tech. 2010 Mar 6; 43 (7): 1128-1131. [ Links ]
21. Adams A, Demyttenaere JCR, De Kimpe N. Biotransformation of (R)-(+)- and (S)-(-)-limonene to a-terpineol by Penicillium digitatum investigation of the culture conditions. Food Chem. 2002 Sept 19; 80 (4): 525-534. [ Links ]
22. Badee AZM, Helmy SA, Morsy NFS. Utilisation of orange peel in the production of a-terpineol by Penicillium digitatum (NRRL 1202). Food Chem. 2010 Nov 19; 126 (3): 849-854. [ Links ]
23. Tan Q, Day DF, Cadwallaer KR. Bioconversion of (R)-(+)- limonene by P. digitatum (NRRL 1202). Process Biochem. 1998 Apr 27; 33 (1): 29-37. [ Links ]
24. Tan Q, Day DF. Bioconversion of limonene to a-terpineol by immobilized Penicillium digitatum. Appl Microbiol Biot. 1997; 49 (1): 96-101. [ Links ]
25. Tan Q, Day DF. Organic co-solvent effects on the bioconversion of (R)-(+)-limonene to (R)-(+)-a-terpineol. Process Biochem. 1998 Feb 8; 33 (7): 755-761. [ Links ]
26. Pescheck M, Mirata MA, Brauer B, Krings U, Berger RG, Schrader J. Improved monoterpene biotransformation with Penicillium sp., by use of closed gas loop bioreactor. J Ind Microbiol Biot. 2009 Mar 26; 36 (6): 827-836. [ Links ]
27. Abraham WR, Hoffmann HMR, Kieslich K, Reng G, Stumpf B. Microbial transformation of some monoterpenoids and sesquiterpenoids. Toronto, Canada: John Wiley & Sons. 1985. 160 p. [ Links ]
28. Cadwallader KR, Braddock RJ, Parish ME, Higgins DP. Bioconversion of (4R)-(+)- limonene by Pseudomonas gladioli. J Food Sci. 1989; 54 (5): 1241-1245. [ Links ]
29. Cadwallader KR, Braddock RJ. Enzymatic hydration of (4R)- (+)-limonene to (4R)-(+)-a-terpineol. Dev Food Sci. 1992; 29: 571-584. [ Links ]
30. Savithiry N, Cheong TK, Oriel P. Production of a-terpineol from Escherichia coli cells expressing thermostable limonene hydratase. App Biochem Biotech.1997 Mar-Jun; 63-65: 213-220. [ Links ]
31. Bicas LJ, Fontanille P, Pastore G M, Larroche C. A bioprocess for the production of high concentrations of R-(+)-a-terpineol from R-(+)-limonene. Process Biochem. 2009 Nov 13; 45 (4): 481-486. [ Links ]
32. Trinci APJ. A kinetic study of the growth of Aspergillus nidulans and the other fungi. J Gen Microbiol. 1969 Jul; 57 (1): 11-24. [ Links ]
33. Pirt SJ. Principles of microbes and cell cultivation. Oxford, England: Blackwell Scientific Publications; 1975. 268p. [ Links ]
34. Pandey DK, Tripathi NN, Tripathi RD. Fungitoxic and phytotoxic properties of the essential oil of H. suaveolens. Zeit Pf lazenran Pflanzensch. 1982; 89: 344-349. [ Links ]
35. Eng F, Gutiérrez M, Favela E. A survey of temperature and pH effect on colonial growth of Botryodiplodia theobromae RC1. Rev Iberoam Micol. 2003 Dec; 20 (4): 172-175. [ Links ]
36. Demyttenaere JCR, Van Belleghem K, De Kimpe N. Biotransformation of (R)-(+)- and (S)-(-)-limonene by fungi and the use of solid phase microextraction for screening. Phytochemistry. 2001 Apr 24; 57 (2): 199-208. [ Links ]
37. Muñoz J, Ros R, Arrillaga I, Segura J. Expression of spearmint limonene synthase in transgenic spike lavender results in an altered monoterpene composition in developing leaves. Met Eng. 2008 Apr 17; 10 (3-4): 166-177. [ Links ]