Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.18 no.2 Medellín May/Aug. 2011
PHARMACOLOGY AND TOXICOLOGY
CYTOTOXIC LABDANE DITERPENOIDS ISOLATED FROM THE HEXANE FRACTION OF THE Croton stipuliformis STEM BARK
DITERPENOIDES LABDANOS CITOTÓXICOS AISLADOS DE LA FRACCIÓN HEXÁNICA DE LA CORTEZA DE Croton stipuliformis
Martha S. FRANCO1, Claudia P. CORDERO2, Sandra J. MORANTES2, Fabio ARISTIZABAL2, Coralia OSORIO3
1 Departamento de Química. Universidad Nacional de Colombia. AA 14490 Bogotá, Colombia.
2 Departamento de Farmacia. Universidad Nacional de Colombia. AA 14490 Bogotá, Colombia.
3 Departamento de Química. Universidad Nacional de Colombia. AA 14490 Bogotá, Colombia. cosorior@unal.edu.co.
Received: 02 September 2010 Accepted: 18 July 2011
ABSTRACT
The composition of the cytotoxic hexane fraction of the Croton stipuliformis bark was studied by means of a gas chromatography coupled to a mass spectrometry; sesquiterpenes and sterols were characterized as the main constituents. Along with these compounds, three labdane and seco-labdane diterpenoids, 8(17),12E,14-labdatrien-18-oic acid [3], 12E-3,4-seco-labda-4(18),8(17),12,14-tetraen-3-oic acid [2], and its methyl ester [1] were isolated from this fraction through preparative HPLC, and their structures were elucidated by HR-FABMS, 1D and 2D NMR analyses. Additionally, the cytotoxic activity of these three compounds against human tumor cell lines HEp-2, HT-29, MKN-45, MCF-7, and HeLa was assessed. The three compounds showed a non-specific and moderate cytotoxicity against the abovementioned cell lines.
Keywords: Croton stipuliformis, Euphorbiaceae, labdane, MTT assay, cytotoxicity.
RESUMEN
La composición de la fracción hexánica de la corteza de Croton stipuliformis se estudió por cromatografía de gases acoplada a espectrometría de masas; se caracterizaron los sesquiterpenos y esteroles como los principales constituyentes. Adicionalmente a estos compuestos, tres diterpenoides labdanos y seco-labdanos, el ácido 8(17),12E,14-labdatrien-18-oico [3], el ácido 12E-3,4-seco-labda-4(18),8(17),12,14-tetraen-3- oico [2] y su ester metílico [1] se aislaron de esta fracción por HPLC preparativa y sus estructuras se elucidaron con base en los análisis por HRFABMS, espectroscopía de RMN mono y bidimensional. Adicionalmente, se determinó la actividad citotóxica de estos tres compuestos frente a las líneas celulares de tumores humanos HEp-2, HT-29, MKN-45, MCF-7 y HeLa. Todos los compuestos mostraron una citotoxidad moderada y no específica frente a las líneas celulares mencionadas anteriormente.
Palabras Clave: Croton stipuliformis, Euphorbiaceae, labdano, ensayo MTT, citotoxidad.
INTRODUCTION
Euphorbiaceae is a big family of about 8,000 species that usually grow in tropical regions. In Colombia, this family is comprises 78 genera and 390 species, from which 80 species belong to the Croton genus (1). Croton stipuliformis (Euphorbiaceae) is a species native to Colombia and it grows under mild climates between 500 and 1800 meters above sea level in the Andes area at the west-central region of Colombia. It is a tree that reaches a height of 25- 30 m, and it is commonly known as ''guacamayo,'' ''candelero'' or ''mopo.'' Usually, it is confused with C. cupreatus, a phenotypically close species (2). In the previously mentioned region, this native plant is recognized because its bark produces a yelloworange latex called ''Sangre de drago,'' which is used for different pharmacological purposes, such as ulcer treatment, pain, anaemia, and cancer (3).
The latex exuded by several Croton species has shown cytotoxic activity against various human tumor cell lines (3, 4). Several classes of diterpenoids have been identified be responsible for this activity; among which, labdanes, cembranoids, traquilobanes, pimaranes, ent-kauranes, and clerodanes, are the most remarkable.
As part of our current studies on the bioactive compounds found in Colombian native plants (5-7), the aim of this research was to perform a bioguided fractionation of the hexane fraction from the bark of Croton stipuliformis, in order to isolate and characterize the compounds responsible for its cytotoxic activity.
MATERIALS AND METHODS
General
1H- and 13C NMR (400 and 100 MHz, respectively) spectra were acquired on a Bruker Biospin® 400 spectrometer (Karlsruhe, Germany). NMR spectra were recorded in CDCl3, and referenced to a TMS signal. HRFAB-MS spectra were recorded in a glycerol matrix in the positive ion mode with a Micromass AutoSpec®-Q spectrometer (Manchester, UK). GC-MS and direct inlet-MS (EIMS, 70 eV) were carried out on a Shimadzu® GCMS QP5050 instrument. The analytical HPLC was performed on a Merck-Hitachi® system equipped with a L-6200 A pump, a L-4500 diode array detector and a D-6000 A unit (Darmstadt, Germany); while for the preparative HPLC, a L-4250 UV-Vis detector was used. Silica gel (0.063-0.200 mm, Merck) was used for column chromatography, and the TLC was performed on silica gel GF254 plates (0.10mm, Merck®). Optical rotations were measured with a Polartronic E, Schmidt & Haensch® polarimeter (Berlin, Germany). The solvents were purchased from Merck® (Darmstadt, Germany).
Plant material
The C. stipuliformis stem bark was collected at Chinchiná, Caldas, Colombia. A voucher specimen (COL 512797) was identified by Dr. J. Murillo and deposited at the Instituto de Ciencias Naturales, Universidad Nacional de Colombia.
Plant material extraction
The dried C. stipuliformis stem bark (3.2 kg) was extracted with methanol (1.5 L, two times) at 60ºC during 4 h period of time. The methanol was evaporated in vacuo to obtain a crude extract (207 g). A part of the methanolic extract (115 g) was suspended in water (500 mL) and extracted with dichloromethane (150 mL x 3); the two phases were separated and after their concentration, organic (CSO, 39.6 g) and aqueous (CSA, 52.9 g) fractions were obtained. A portion of the organic fraction (12.4 g) was extracted with hexane (60 mL x 3) to obtain the CSH fraction (5.2 g).
Fractionation and purification of cytotoxic compounds
The bioactive hexane fraction (695 mg) was subjected to open silica gel column chromatography (70 g), sequentially eluting it with 200 mL each of hexane:AcOEt in ratios of 10:0, 7:3, 5:5, 3:7 and 0:10 to afford four pooled fractions as follows: CSHa (15 mg), CSHb (150 mg), CSHc (182 mg), and CSHd (180 mg). The fractions were pooled together based on their similar Rf values on thin layer chromatography (TLC), which was developed with the mobile phase system of hexane:AcOEt (1:1); then, they were sprayed using an anisaldehyde:sulfuric acid solution (1%: 2% v/v) in EtOH, followed by heating at 110°C. All of the fractions were analyzed through GC-MS, and CSHb and CSHc fractions were also analyzed by HPLC-DAD on a SynergiTM 4μ MAX-RP 80A column (4.0 μm, 250 mm x 4.60 mm i.d., Phenomenex®), using a methanol:acetonitrile gradient as mobile phase.
CSHb and CSHc fractions were further subjected to preparative HPLC using a GeminiTM 5μ C18 column (5μm, 250mm x 10mm i.d., Phenomenex®), with methanol:acetonitrile (9:1) as mobile phase at a flow rate of 5 mL/min. Thus, the CSHb fraction only afforded compound 1 (105 mg), while the CSHc fraction yielded compounds 1 (90 mg), 2 (28 mg), and 3 (24 mg).
12E-3,4-seco-labda-4(18),8(17),12,14-tetraen-3- oic acid methyl ester (1) was obtained as a yellow oil. [α]D25 = +13.0° (CH2Cl2; c 0.04). EIMS m/z {rel. int.}: 316 [M+] {10}, 301 [M-CH3]+ {7}, 285 [M-CH3O]+ {5}, 273 [M-C3H7]+ {45}, 259 {7}, 247 {10}, 229 [273-CO2]+ {40}, 219 {10}, 201 [229-CH2CH2]+ {25}, 187 {20}, 173 {40}, 161 {50}, 147 {35}, 145 {42}, 133 {48}, 119 {70}, 105 {60}, 93 {55}, 91 {75}, 81 {80}, 79 {100}, 77 {52}, 67 {58}, 55 {90}, 53 {52}. HRFABMS found 317.2481 [M+H]+, C21H33O2 requires 317.2474. 1H NMR (400 MHz, CDCl3): δ 0.75 (3H, s, H3-20), 1.53- 1.61 (1H, m, H-6a), 1.72 (2H, overlapped, H-6b, H-11a), 1.72 (3H, s, H3-19), 1.74 (1H, overlapped, H-1a), 1.76 (3H, s, H3-16), 1.77 (1H, overlapped, H-1b), 1.88 (1H, br t, J = 6.2 Hz, H-9), 1.99 (1H, dt, J = 12.8, 4.8 Hz, H-7a), 2.19 (1H, overlapped, H-2a), 2.24 (1H, dd, J = 12.0, 3.2 Hz, H-5), 2.25 (1H, overlapped, H-11b), 2.34 (1H, ddd, J = 12.8, 4.0, 2.4 Hz, H-7b), 2.44 (1H, ddd, J = 13.8, 11.6, 5.6 Hz, H-2b), 3.64 (3H, s, OCH3), 4.48 (1H, br s, H-17a), 4.68 (1H, br s, H-18a), 4.85 (1H, br s, H-18b), 4.86 (1H, d, J = 1.8 Hz, H-17b), 4.88 (1H, d, J = 10.8 Hz, H-15a), 5.04 (1H, d, J = 17.4 Hz, H-15b), 5.34 (1H, t, J = 6.0 Hz, H-12), 6.31 (1H, dd, J = 17.4, 10.7, H-14); 13C NMR (100 MHz, CDCl3): δ 12.1 (C-16), 17.6 (C-20), 23.6 (C-11), 23.7 (C-19), 28.1 (C-2), 30.2 (C-6), 32.7 (C-1), 37.7 (C-7), 41.4 (C-10), 49.3 (C-9), 51.0 (C-5), 51.8 (OCH3), 108.7 (C-17), 110.4 (C-15), 113.9 (C-18), 133.2 (C-12), 133.9 (C-13), 141.6 (C-14), 147.2 (C- 4), 147.5 (C-8), 174.4 (C-3).
12E-3,4-seco-labda-4(18),8(17),12,14-tetraen-3- oic acid (2) was obtained as a colorless oil. [α]D25=-55.6° (CH2Cl2; c 0.06). EIMS m/z {rel. int.}: 302 [M+] {10}, 287 [M-CH3]+ {8}, 273 [M-29]+ {5}, 259 [M-C3H7]+ {15}, 247 {3}, 245 {8}, 229 [M-COOHC 2H4]+ {30}, 219 {10}, 203 {20}, 201 {20}, 187 {20}, 173 {23}, 161 {50}, 147 {40}, 133 {45}, 131 {22}, 119 {70}, 107 {67}, 105 {70}, 93 {73}, 91 {75}, 81 {80}, 79 {100}, 77 {49}, 69 {39}, 67 {39}, 65 {25}, 55 {70}, 53 {48}. HRFABMS found 301.1233 [M-H]+, C20H29O2 requires 301.1229. 1H NMR (400 MHz, CDCl3): δ 0.75 (3H, s, H3-20), 1.58 (1H, m, H-6a), 1.71 (1H, overlapped, H6b), 1.72 (1H, overlapped, H-11a), 1.72 (3H, s, H3-19), 1.74 (1H, overlapped, H-1a), 1.75 (3H, br s, H3-16), 1.76 (1H, overlapped, H-1b), 1.87 (1H, br t, J = 6.8 Hz, H-9), 1.98 (1H, dt, J = 12.8, 5.2 Hz, H-7a), 2.20-2.24 (1H, m, H-11b), 2.21 (1H, dd, J = 12.4, 3.6 Hz, H-5), 2.25 (1H, ddd, J = 15.2, 12.4, 5.6 Hz, H-2a), 2.34 (1H, ddd, J = 12.8, 4.0, 2.4 Hz, H-7b), 2.46 (1H, ddd, J = 15.2, 12.0, 5.2 Hz, H-2b), 4.47 (1H, br s, H-17a), 4.68 (1H, br s, H-18a), 4.85 (1H, br s, H-18b), 4.86 (1H, d, J = 1.8 Hz, H-17b), 4.88 (1H, d, J = 10.4 Hz, H-15a), 5.04 (1H, d, J = 17.6 Hz, H-15b), 5.33 (1H, t, J = 6.4 Hz, H-12), 6.30 (1H, dd, J = 17.2, 11.2, H-14); 13C NMR (100 MHz, CDCl3): δ 12.0 (C-16), 17.5 (C-20), 23.4 (C-11), 23.5 (C-19), 28.0 (C-2), 30.0 (C-6), 32.3 (C-1), 37.5 (C-7), 41.2 (C-10), 49.2 (C-9), 50.8 (C-5), 108.6 (C-17), 110.3 (C-15), 113.8 (C-18), 132.8 (C-12), 133.9 (C-13), 141.4 (C-14), 147.0 (C-8), 147-2 (C-4), 180.1 (C-3).
8(17),12E,14-labdatrien-18-oic acid (3) was obtained as a white solid. [α]D25= -15.4° (CH2Cl2; c 0.03). EIMS m/z {rel. int.}: 302 [M+] {25}, 287 [MCH3]+ {40}, 273 [M-29]+ {10}, 257 [M-COOH]+ {10}, 246 {38}, 241 {22}, 231 {27}, 221 {8}, 201 {20}, 185 {15}, 175 {70}, 161 {20}, 147 {48}, 135 {53}, 134 {45}, 121 {52}, 119 {70}, 107 {55}, 105 {54}, 93 {75}, 91 {80}, 81 {90}, 79 {100}, 77 {40}, 67 {43}, 55 {52}, 53 {45}. The HRFABMS gave 301.2149 [M-H]+ as result, which is compatible with the molecular formula C20H29O2. 1H and 13C-NMR data were in agreement with those previously published by Smith et al., 2007 (8).
GCMS analyses
HRGC-EIMS analyses were carried out on a RTX-5 column (30 m x 0.25 mm i.d., 0.25 μm film thickness). The column oven was maintained at 60°C for 4 min and then programmed to increase the temperature from 60 to 300°C at 4°C/min. The f inal temperature was held for 15 min, and the injector temperature was maintained at 300°C. Helium was used as carrier gas at 1.0 mL/min; injection volume was 1 μL in split mode (1:10). MS data were recorded in a mass range of 30 - 500 U, with an electron energy of 70 eV, and they were processed by a Class 5000 v 2.2 MS-Workstation® software. The CSHa fraction was also analyzed on a DB-Wax column (30 m x 0.25 mm i.d., 0.25 μm film thickness). The injector port temperature was maintained at 230°C and the split ratio was set to 1:10. The oven temperature was initially fixed at 50°C for 4 min, then it was increased at 4°C/min until reaching 200°C, and then it was maintained at that level for 10 min. Helium was the carrier gas used at 1.0 mL/min.
Kovats retention indices on the two previously described different columns were calculated and compared with the indices found in the literature values (9). The constituents of the CSHa and CSHd fractions were identified by matching their mass spectra with those from known databases (10-12).
Biological evaluation
A bioassay of the cytotoxic activity against human tumor cell culture in vitro was performed by means of the MTT (3-(4,5-dimethyl-2-thiazolyl)- 2,5-diphenyl-2H-tetrazolium bromide) colorimetric assay (13, 14). Cancer cell lines of human larynx (HEp-2), colon (HT-29), gastric (MKN-45), breast (MCF-7) and cervical cells (HeLa) were obtained from the American Type Culture Collection (ATCC®, Rockville, MD). The cells were cultured in MEM (Minimum Essential Medium, SIGMA®) supplemented with 5% fetal bovine serum (Vitacell, ATCC, VA) and gentamycin (50 μg/mL).
For the experiments, the cells were plated in 100 μL of culture medium in 96-well; after 24 h, the fractions and pure compounds (1 - 3) were added to each well at different concentrations (50, 5, and 0.5 μg/mL in DMSO), and then they were incubated for 48 h in a total of 200 μL of medium volume. Each treatment was evaluated in triplicate. At the end of the treatment, the medium in each well was replaced with fresh medium (100 μL) containing 0.25 mg/mL of MTT. Four hours later, the formazan product of the MTT reduction was dissolved in 100 μL of DMSO, and absorbance was read at 570 nm on a BIORAD 550 spectrophotometer. The percentages of cell survival related to the growth control wells (wells containing only cells and medium) were calculated, and the LC50 (concentration that reduces the exposed sample survival rate to 50%) was determined. Doxorubicin hydrochloride was used as a positive control substance; for this purpose, four serial dilutions were prepared at a maximum concentration of 10 μm (5.8 μg/mL).
Statistical analysis
Data were expressed as the mean values ± the standard error (SE). GraphPad–Prism® 4.0 was used to compare LC50 values by means of the ANOVA analysis, followed by the Tukey Test, with a 95% of confidence.
Computational methods
All computations were carried out with the GAMESS program package (version 2006, Gordon Research Group, Iowa State University, USA).
RESULTS AND DISCUSSION
A preliminary screening of various doses of crude methanol extract of C. stipuliformis indicated its activity against five human cancer cell lines. Following the partitioning of the MeOH extract, the cytotoxic constituents were identified in the organic fraction (CSO) with LC50 values below or near to 100 mg/mL (15); while the aqueous extract exhibited no significant inhibition of growth in the population of the tested cancer cells.
The GC-MS analysis of the C. stipuliformis bark hexane fraction revealed that CSH contained diterpenoid compounds (75.0%), sterols (16.0%), aliphatic esters (5.4%), sesquiterpenes (1.8%), aliphatic alcohols (1.6%), and other types of compounds (0.2%). Further fractionation of hexane fraction was needed due to its complexity. It afforded four fractions (i.e. CSHa-d), which were also analyzed through GC-MS, as it can be seen in figure 1. This analysis allowed determining that the composition between fractions was different as a result of an efficient separation; thus, the first CSHa fraction contained mono- and sesquiterpenoids; CSHb and CSHc showed the presence of diterpenoids; and CSHd consisted mainly of triterpenes and sterols.
The careful analysis of mass spectra and retention indexes of CSHa constituents allowed the identification of 51 compounds in this fraction, which identity is presented in table 1. The major constituents were identified as follows: the sesquiterpenes β-cariophyllene (10.48%), δ-cadinene (7.72%), γ-muurolene (3.81%), and α-copaene (2.25%); the diterpene 1 (6.73%); aliphatic esters methyl hexadecanoate (8.86%) and methyl octadecanoate (3.82%); butanol (18.39%), and heneicosane (7.56%). This high terpenoid content is comparable with those found for other Croton species, such as C. zambesicus (16) and C. sellowii (17).
The CSHd fraction was mainly constituted by sterols, among which β-sitosterol (34.94%), β-amirene (17.70%), ergosta-5-en-3-ol (11.24%), and ergosta-4-en-3-one (5.17%) were the major constituents. It is important to point out that most of the identified sterols have Δ5-nuclei, which is common in the Croton species (4).
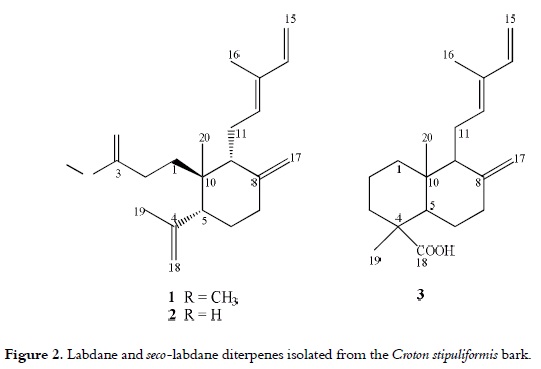
The strongest cytotoxic activities were detected for CSHb and CSHc, which were subsequently fractionated by means of preparative HPLC in order to obtain pure compounds 1-3. The major constituent of the CSHb fraction (1) has a formula of C21H32O2, as it was established through the HRFABa Composition calculated based on the peak area % of each fraction. b The eight most intense peaks of MS. - Not detected. MS analyses. The 13C NMR spectra confirmed that compound 1 possesses a carboxyl group (δC 174.4). Also, the 1H NMR spectra suggested that compound 1 possesses a –CH=C(CH3)-CH=CH2 group, which is typical for a labda-12(E),14-diene skeleton (18, 19), along with the presence of a cyclohexane ring, which was determined by of COSY and HMBC correlations. An exomethylene group was shown by the 1H and 13C NMR signals at δH 4.48 (H-17a, brs) and 4.86 (H-17b, d, J = 0.8), and δC 108.7 (C-17). The 1H NMR spectrum also showed two additional singlet due to the presence of two methyl groups at δH 0.75 (H3-20) and 1.72 (H3-19); the latter was attached to an olef inic group, which was attached to the cyclohexane ring. Therefore, the carboxyl group must be confined to an aliphatic chain attached to the cyclohexane system, which could be probed by the correlations between the carboxylic carbon at δC 174.4 (C-3) and methylene protons at δH 2.19 (H-2a) and 2.44 (H-2b) observed in the HMBC spectrum. These correlations revealed that the structure of compound 1 resembles a 3,4-seco-labda-12(E),14-diene skeleton due to the ring A opening; thus, it was elucidated as the methyl ester of (+)-12E-3,4-seco-labda- 4(18),8(17),12,14-tetraen-3-oic acid (maravuic acid methyl ester). The chemical structure of this compound is presented in figure 2.
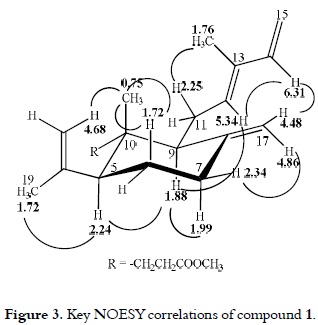
As it can be seen in figure 3, the relative stereochemistry of the substituents at the cyclohexane ring was proposed based on the analysis of the NOESY spectrum. The 1H NMR spectrum of compound 1 clearly showed an axial configuration (δ2.24, dd, W½ = 21 Hz) for H-5. Irradiation of methyl protons at δH 0.75 (H3-20) enhanced the methylene proton signal at δH 1.72 (H-6ax and H-11a), 2.25 (H-11b) and 4.68 (H-18a). The irradiation of H-9 (δH 1.88) enhanced the proton signals of H-5 (δH 2.24) and H-7ax (δH 1.99), thus revealing a trans-1,3-diaxial configuration of H-5, H-7ax, and H-9 protons. Further NOESY correlation between H-5 and H3-19 (δH 1.72) was also found. Thus, it was possible to establish that the cyclohexane ring has a chair conformation with the isopropenyl group at C-5 and the side-chain at C-9 in a β-equatorial orientation. (E)-Geometry of the C-12 double bound was evident from the upfield chemical shift of the methyl group at C-13 (δH 1.76, δC 12.1), and it was confirmed by the correlations between H-12 (δH 5.34) and H-14 (δH 6.31), as well as, the correlations between H3-16 (δH 1.76) and H-11b (δH 2.25) in the NOESY spectrum. Finally, the correlation between the olefinic proton H-17b (δH 4.86) and H-7b (δH 2.34) suggests an equatorial orientation for this proton. The compound 1 structure optimization, which was done using the GAMESS computer program (20), is presented in figure 3. It is notable that compound 1 can be considered as an artifact of the MeOH reflux because the acid was also isolated.
1H and 13C NMR spectral data for compound 2 resembles those of compound 1, except for the presence of a methoxy group (δH 3.64, δC 51.8). Therefore, compound 2 was identified as the 12E-3,4-seco-labda-4(18),8(17),12,14-tetraen-3- oic acid or Maravuic acid, a seco-labdane diterpene previously isolated from Croton stipuliformis leaves (21) and Croton matourensis bark (22).
The molecular formula of compound 3 was deduce as C20H30O2 based on the HRFAB-MS and the EIMS [M+] (m/z 302). The unequivocal assignment of compound 3 was established through the analysis of data from the HMQC, HMBC, and COSY experiments. The spectral data of the decalin ring system with 8(17) exomethylene of this compound was in agreement with those of the 12,15-epoxy-8(17),13-labdadienoic acid isolated from Chunninghamia konishii (23). Thus, compound 3 was identified as 8(17),12E,14-labdatrien-18-oic acid (communic acid), which has been previously reported in Xylopia langsdorffiana (24), Isodon lophanthoides (25) and Pinus resinosa (26), among others. The cardiovascular effects of compound 3 as hypotensor have been reported before (27); however, this is the first study about its cytotoxic activity.
The cytotoxicity of Croton stipuliformis bark methanol extract against human tumor cell lines were found in the organic fraction (CSO), as well as in the hexane fraction (CSH); as it was expected, crude methanol extract showed lower inhibitory effects compared to the subsequent fractions. The pure compounds (1-3) were evaluated for cytotoxicity against human larynx adenocarcinoma (HEp-2), human colon adenocarcinoma (HT-29), human gastric carcinoma (MKN-45), human breast carcinoma (MCF-7), and human cervix carcinoma (HeLa); and doxorubicin was used as positive control. The results for the afore mentioned evaluations are presented in table 2. All three diterpenes showed non-specific moderate cytotoxicities against those cell human tumor lines.
Compound 1 showed a weak activity against human larynx adenocarcinoma (47.2 μg/mL) in comparison to the other human tumour lines tested, which can be interpreted as a slight selectivity towards the growth of cancer cell lines MCF-7, HeLa, HT-29 and MKN-45. Analogue compound 2 showed higher cytotoxic activity values than those of compounds 1 and 3. It is very interesting to see that compounds 2 and 3 were more active than compound 1; therefore, it is possible to establish that the presence of the methyl ester group diminished the cytotoxicity of compound 1. This behaviour was also found for the benzyl ester of compound 2, which was synthesized as part of this work (data not shown), and allowed us to confirm the significance of acid moiety in cytotoxicity.
It is important to point out that the cytotoxic activity of the three diterpenes isolated from Croton stipuliformis is higher than other known diterpenes, usually found in the Croton genus, such as crotonin and dehydrocrotonin (28-30).
CONCLUSIONS
The hexane fraction of Croton stipuliformis presented a moderate cytotoxic activity against different in vitro human cancer cell lines. Moreover, the cytotoxicity of diterpenes 1-3 isolated from the afore mentioned species was demonstrated for the first time in this study.
ACKNOWLEDGMENTS
Financial support from DINAIN-Universidad Nacional de Colombia is greatfully acknowledged. The authors are grateful to Dr. Ricardo Acuña from CENICAFE (Colombia) for providing us with the sample for this study. The authors also would like to thank Prof. Dr. Francisco José Heredia from Universidad de Sevilla for his support on the recording of HRMS experiments.
REFERENCES
1. Murillo JA. Las Euphorbiaceae de Colombia. Biota Colombiana. 2004 Dec; 5 (2): 183-200. [ Links ]
2. Murillo JA. Composición y distribución del género Croton (Euphorbiaceae) en Colombia, con cuatro especies nuevas. Caldasia. 1999 Sep; 21 (2): 141-166. [ Links ]
3. Jones K. Review of Sangre de Drago (Croton lechleri)-a south american tree sap in the treatment of diarrhea, inflammation, insect bites, viral infections, and wounds: traditional uses to clinical research. J Altern Complem Med. 2003 Dec; 9 (6): 877-896. [ Links ]
4. Salatino A, Faria Salatino ML, Negri G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J Braz Chem Soc. 2007 Jan-Feb; 18 (1): 11-33. [ Links ]
5. Sagawa T, Takaishi Y, Fujimoto Y, Duque C, Osorio C, Ramos F, et al. Cyclobutane dimers from the Colombian medicinal plant Achyrocline bogotensis. J Nat Prod. 2005 Mar; 68 (4): 502-505. [ Links ]
6. Ramos F, Takaishi Y, Kawazoe K, Osorio C, Duque C, Acuña R, et al. Immunosuppressive diacetylenes, ceramides and cerebrosides from Hydrocotyle leucocephala. Phytochemistry. 2006 Jun; 67 (11): 1143-1150. [ Links ]
7. Cordero CP, Morantes SJ, Páez A, Rincón J, Aristizábal FA. Cytotoxicity of withanolides isolated from Acnistus arborescens. Fitoterapia. 2009 Sep; 80 (6): 364-368. [ Links ]
8. Smith ECJ, Williamson EM, Wareham N, Kaatz GW, Gibbons S. Antibacterials and modulators of bacterial resistance from the immature cones of Chamaecyparis lawsoniana. Phytochemistry. 2007 Jan; 68 (2): 210-217. [ Links ]
9. Kondjoyan N, Berdagué JL. A compilation of relative retention indices for the analysis of aromatic compounds. Theix, France: Laboratoire Flaveur; 1996. [ Links ]
10. McLafferty FW, Stauffer D B. The Wiley/NBS Registry of Mass Spectral Data, 5th ed. New York, United States: Wiley; 1991. [ Links ]
11. Joulain D, König WA. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons. Hamburg, Germany: Verlag; 1998. [ Links ]
12. Adams RP. Identification of essential oil components by gas chromatography/Quadrupole Mass Spectroscopy. Carol Stream, IL, United States: Allured Publishing Corporation, 2001. [ Links ]
13. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods. 1983 Dec; 65 (1-2): 55-63. [ Links ]
14. Cordero C, Aristizábal F. Evaluación preliminar in vitro de citotoxicidad de extractos vegetales, empleando métodos colorimétricos. Rev Col Biotecn. 2002 Jan-Feb; 4 (1): 100-106. [ Links ]
15. Teicher, B., Totowa, N. J. Eds, Anticancer drug development guide: preclinical screening, clinical trials and approval. New York, USA: Humana Press, 1997. Boyd M. The NCI in vitro anticancer drug discovery screen: concept, implementation and operation 1985-1995; p. 23-42. [ Links ]
16. Fekam Boyom F, Keumedjio F, Jazet Dongmo PM, Ngadjui BT, Amvam Zollo PH, Menut C, et al. Essential oils from Croton zambesicus Muell. Arg. growing in Cameroon. Flavour Frag J. 2002 May-Jun; 17 (3): 215-217. [ Links ]
17. Conserva EM, Palmeira JSF, Moura F, Alves V, Oliveira FM, Bento ES, et al. Neutral components from hexane extracts of Croton sellowii. Flavour Frag J. 2004 Jan-Feb; 19 (1): 69-71. [ Links ]
18. Roengsumran S, Petsom A, Sommit D, Vilaivan T. Labdane diterpenoids from Croton oblongifolius. Phytochemistry. 1999 Feb; 50 (3): 449-453. [ Links ]
19. Noma M, Suzuki F, Gamou K, Kawashima N. Two labdane diterpenoids from Nicotiana raimondii. Phytochemistry. 1982 Feb; 21 (2): 395-397. [ Links ]
20. Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, et al. General atomic and molecular electronic structure system. J Comput Chem. 1993 Nov; 14 (11): 1347-1363. [ Links ]
21. Ramos FA, Takaishi Y, Kashiwada Y, Osorio C, Duque C, Acuña R, et al. Ent-3,4-seco-labdane and ent-labdane diterpenoids from Croton stipuliformis (Euphorbiaceae). Phytochemistry. 2008 Sep; 69 (12): 2406-2410. [ Links ]
22. Schneider C, Breitmaier E, Bayma J, de Franca LF, Kneifel H, Krebs HC. Maravuic acid, a new seco-labdane diterpene from Croton matourensis. Liebigs Ann. 1995 Mar; (4): 709-710. [ Links ]
23. Li YC, Kuo YC. Labdane-type diterpenoids from the wood of Chunninghamia konishii. Chem Pharm Bull. 2002 Apr; 50 (4): 498-500. [ Links ]
24. Ribeiro LA, Tavares JF, De Andrade N, Da Silva M, Da Silva B. The (8)17,12E,14-labdatrien-18-oic acid (labdane 302), a labdane-type diterpene isolated from Xylopia langsdorffiana St. Hil. & Tul. (Annonaceae), relaxes the guinea pig trachea. Rev Bras Farmacogn. 2007 Jun; 17 (2): 197-203. [ Links ]
25. Jiang B, Lu ZQ, Zhang HJ, Zhao QS, Sun HD. Diterpenoids from Isodon lophanthoides. Fitoterapia. 2000 Aug; 71 (4): 360-364. [ Links ]
26. Zinkel DF, Clarke WB. Resin acids of Pinus resinosa needles. Phytochemistry. 1985 Jun; 24 (6): 1267-1271. [ Links ]
27. Pereira de Oliveira A, Furado F, Sobral da Silva M, Tavares J, Mafra RA, Araujo DA, et al. Calcium channel blockade as a target for the cardiovascular effects induced by the 8(17),12E,14- labdatrien-18-oic acid (labdane-302). Vasc Pharmacol. 2006 May; 44 (5): 338-344. [ Links ]
28. Rodríguez JA, Haun M. Cytotoxicity of trans-dehydrocrotonin from Croton cajucara on V79 cells and rat hepatocytes. Planta Med. 1999 Aug; 65 (6): 522-526. [ Links ]
29. Haun M, Conte Anazetti M, Silva Melo P, Durán N. Comparative cytotoxicity of dimethylamide-crotonin in the promyelocytic leukemia cell line (HL60) and human peripheral blood mononuclear cell. Toxicology. 2003 Jun 30; 188 (2-3): 261-274. [ Links ]
30. Grynberg NF, Echevarria A, Lima JE, Pamplona SSR, Pinto AC, Maciel MAM. Anti-tumour activity of two 19-nor-clerodane diterpenes, trans-dehydrocrotonin and trans-crotonin, from Croton cajucara. Planta Med. 1999 Dec; 65 (8): 687-689. [ Links ]