Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.18 no.2 Medellín May/Aug. 2011
PHARMACEUTICAL INDUSTRY
VACCINE FORMULATION: ADSORPTION OF Plasmodium falciparum MSP-1 PEPTIDE 1585 ON ALUMINIUM HYDROXIDE
FORMULACIÓN DE VACUNAS: ADSORCIÓN SOBRE HIDRÓXIDO DE ALUMINIO DEL PEPTIDO 1585 DERIVADO DE LA PROTEINA MSP-1 DE Plasmodium falciparum
Mary TRUJILLO1*; Luz M. SALAZAR2; Jesús VALENCIA2
1 Facultad de Ciencias, Universidad Nacional de Colombia, sede Bogotá. Carrera 30 No. 45-03, Tel.: +57-1-3165000 ext. 14620 Fax: +57- 1-3165000 ext. 14640. Bogotá, Colombia. mtrujillog@unal.edu.co.
2 Facultad de Ciencias, Universidad Nacional de Colombia, sede Bogotá. Carrera 30 No. 45-03, Tel.: +57-1-3165000 ext. 14620 Fax: +57- 1-3165000 ext. 14640. Bogotá, Colombia.
Received: 17 January 2011; Accepted: 25 May 2011
ABSTRACT
The Plasmodium falciparum merozoite surface protein 1 has been studied due to its potential to become a vaccine; likewise, the peptide 1585 which is located in the 42-kDa amino-terminal fragment induces protective immunity in primates. Despite the importance of antigen adsorption in the formulation and production of vaccines containing aluminium adjuvant, the protein fragment adsorption on aluminium hydroxide has not been thoroughly studied. Electrostatic attraction, hydrophobic interaction and ligand exchange have been identified as the major mechanisms involved in antigen retention on the adsorbent surface. Peptide 1585 was synthesized, and its solubility, adsorption on aluminium hydroxide, as well as its molecule release have been studied here. Results allowed us to raise a model for the adsorption and release of this peptide, which are important parameters to establish optimal conditions for peptideadsorbent interaction and, therefore, their response as a vaccine. Results also established the reversibility of such process due to the phosphate ion effect. Thus, this work provides a starting point for research works, leading to further development of vaccine formulations containing highly purified synthetic antigens adsorbed on aluminium adjuvant.
Keywords: Adsorption, aluminium hydroxide, peptide, vaccine, Plasmodium falciparum.
RESUMEN
La proteína de superficie del merozoito de Plasmodium falciparum, MSP-1, es reconocida como candidata a vacuna; asimismo, el péptido 1585, situado en la región amino terminal de 42- KDa, induce inmunidad protectiva en primates. A pesar de la importancia de la adsorción del antígeno en la formulación y producción de vacunas que contienen hidróxido de aluminio como adyuvante, la adsorción del fragmento proteíco no ha sido estudiada. Los principales mecanismos que se han identificado como responsables de la retención de un antígeno sobre la superficie adsorbente son las interacciones electrostáticas, las interacciones hidrofóbicas y el intercambio de ligando. En este trabajo se sintetizó el péptido 1585 para estudiar la solubilidad, la adsorción sobre hidróxido de aluminio y la liberación de la molécula. Los resultados permitieron plantear el modelo para la adsorción y la liberación de este péptido, parámetros importantes para establecer las condiciones óptimas para la interacción péptido- adsorbente y por ende, su respuesta como vacuna. Los resultados también mostraron la reversibilidad del proceso debido al efecto del ión fosfato.
Palabras clave: adsorción, hidróxido de aluminio, péptido, vacuna, Plasmodium falciparum.
INTRODUCTION
Most synthetic or recombinant vaccines have shown to be safe; however, their reduced immunogenicity entails the use of immune adjuvants capable of amplifying and directing the host immune response against the antigen.
Aluminium hydroxide (AH) and aluminium phosphate (AP) containing adjuvants are known as deposition adjuvants, since they adsorb the antigen to increase their biological and immunological half-life, and they can be detected by the immune system due to their gradual release.
Studying the parameters that govern peptide adsorption and release onto AH will lead to establishing the necessary conditions for achieving the best adsorbate-adsorbent interaction and, thus, optimizing such process and the vaccine formulation.
The mechanisms underlying peptide adsorption in vaccines formulated with AH are studied by plotting adsorption isotherms at different solution concentrations, traditionally determined by decreasing the quantity of antigen present in the solution.
Protein adsorption studies on AH (1-3) have shown that these molecules are retained according to Langmuir's adsorption model (4), which poses that all adsorption sites are energetically equivalent, that no intermolecular interaction occurs in the system, and that adsorption is accompanied by monolayer formation.
Langmuir's equation has been used as a semi-quantitative approach for characterising physicochemical adsorption parameters, such as adsorption capacity and adsorption coefficient (Kd). These parameters have been successfully applied to predict the competitive effect with other proteins, which should be taken into account at the moment of manufacturing multi-component vaccines adsorbed on AH (5-7).
Other studies have shown that intra and intermolecular interactions may occur depending on the protein structure, causing the formation of multiple antigen layers on the adsorbent surface, which is a situation further favoured at high protein concentrations (2, 5-9).
Models for interpreting the characteristics of adsorption isotherms from solutions describe monolayer or bilayer formation; however, such scheme differs from recent proposals suggesting molecule aggregation on the adsorbent surface.
Protein adsorption on solids is usually irreversible and there is no significant loss of retained protein; however, this condition may be altered when the surface remains immersed in dissolution media.
When the dissolution does not result in protein desorption, proteins may be released from the surface by adding other molecules that can be retained on the surface through an exchange mechanism, in which the adsorption of higher affinity molecules favours polypeptide replacement. Such adsorbed molecule exchange mechanism can be heteromolecular (i.e. proteins displaced by another protein) or homomolecular (i.e. in systems where adsorbed and dissolved molecules are dynamically exchanged between adsorbed and dissolved states) (10).
Depending on the system, the exchange may take minutes or even days and the protein being released does not necessarily keep the same structural characteristics it exhibited before being adsorbed. Adsorption may thus be reversible or irreversible, depending on the protein structure before and after being adsorbed (11).
Antigens adsorbed on aluminium containing adjuvants are exposed to two different environments: the vaccine's components before they are administered, and the interstitial fluid following intramuscular or subcutaneous administration. Many substances contained in the interstitial fluid, such as phosphate (12), citrate ions (13, 14) and interstitial proteins (15) can rapidly alter the degree of antigen adsorption on AH.
Peptide 1585 (1E2V3L4Y5L6K7P8L9A10G11V12Y13R14S15L16K17K18Q19L20E) adsorption was studied in this work; it is a mainstream malaria vaccine candidate derived from Plasmodium falciparum MSP1. It was found that peptide adsorption on AH generated complex isotherms, suggesting the formation of several layers on the adsorbent. A model of the structure of the adsorbed layer is proposed, constituting one of the first kinetic experimental results reported, which are aimed to understanding the molecular interactions between the peptide vaccine candidate and the adsorbent.
MATERIALS AND METHODS
Peptide 1585 synthesis and characterisation
Peptide 1585 was obtained by the solid-phase multiple peptide synthesis method proposed by 185 Merrifield, 1963 (16) and improved by Houghten, 1985 (17). Crude peptide was purified by RPHPLC. Peptide purity was verified on an analytical Lichrosorb® C18 column using 0.05% TFA in water (solvent A), 0.05% TFA in ACN (solvent B), and a 0 - 70% gradient of solvent B for 30 min. Peptide molecular mass was determined on a Bruker MALDI-TOF mass spectrometer. The peptide 1585 secondary structure was assessed by circular dichroism (CD) spectroscopy using a JASCO® spectropolarimeter calibrated with d-10-camphorsulphonic acid; and a 0.1 mM solution of purified peptide was used in 30% TFE-H2O at 298 K.
Adsorption isotherms on AH
0.5 to 12 mg/mL (0.2 - 5 mmol/L) concentration peptide solutions were prepared at constant temperature (273 K) in 0.9% sodium chloride with a 7 ± 0.1 pH. An AH equivalent to 1.6 mg of Al/mL (18) was added, shaking the mixture for 12 hours at 150 rpm. Peptide concentration, before and after adsorption was determined by spectrophotometry at 570 nm using bicinchoninic acid (BCA) (19-21). The amount adsorbed in mmol/mg Al was determined by the difference between these values, and it was plotted in terms of the initial solution concentration.
Release isotherms
Peptide 1585 adsorbed on AH at a 1 mmol/L concentration in aqueous solution was stored for 24 hours at 277 K; a 100 mM sodium phosphate solution (pH 7.0) was then added to obtain a final phosphate ion concentration of 4 mM (12). The mixture was stirred at 310 K and supernatant placed in aliquots, replacing the solvent to obtain a constant volume. The samples were spun at 8,000 rpm for 5 min and the amount of peptide in the solution was determined, using the standard micro-BCA protocol (Pierce) (19 - 21) and the purified peptide pattern curve.
Molecular docking
Docking was performed with a molecular calculation package (2000, Accelrys, San Diego, CA) on an Indigo II work station (Silicon Graphics), using the previously obtained NMR structural model (22), and calculating Van Der Waals energy (VDWE), electrostatic or Coulomb energy (CE), and the combination of both or total energy (TE).
RESULTS AND DISCUSSION
Peptide characterisation
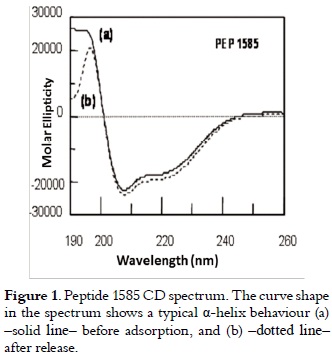
The chromatographic analysis of peptide 1585 in pure state, gave as result a retention time of 23.6 minutes, and mass spectrum showed a 2,348.8 Dalton signal, which corresponds to the expected peptide molecular mass (data no shown). The secondary structure elements forming peptide 1585 tridimensional structure are shown in the DC spectrum in figure 1. The curve (a) (solid line) has a maximum molar ellipticity at 193 nm (transition π - π* positive with Θ in 30,000), and a minimum molar ellipticity at 208 nm (transition π - π* negative and 222 nm transition n-π*), which is characteristic of a helicoidal structure (23).
Adsorption isothermss
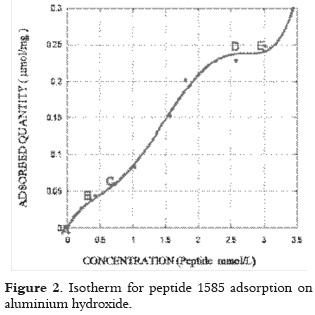
Peptide 1585 adsorption showed low adsorbed peptide solution concentration between A and B (as seen in figure 2), a small increase in adsorption with an attenuation of around 0.5 mmol/L between B and C. Peptide adsorption increased remarkably with concentration, producing a 230% increase in the 1.1 to 2.6 mmol/L range (as shown in D). Adsorption increased slowly between D and E for higher peptide concentrations. Above 3 mmol/L, the adsorbed amount increased even more due to possible peptide aggregation in experimental conditions.
This pattern showed that peptide molecules can cause reduced system entropy, producing a complex isotherm which could not be fully interpreted throughout the whole range of concentrations studied with Langmuir's model.
Initial AH saturation was produced to form the monolayer at concentrations below 0.5 mmol/L; then, a second arrangement of peptide molecules in solution occurred on the adsorbed molecules or double layer between 0.5 and 2.6 mmol/L. If such interpretation is correct, the isotherm can be separated into two independent concentration zones to apply Langmuir's model.
It was found that this model correctly interpreted peptide adsorption if Langmuir's equation (equation 1) applies to both areas, i.e. the area where the monolayer formation is supposed to occur, and in the 2 to 3 mmol/L range.

In equation 1, m is the adsorbed amount of peptide (µmol/mg Al), b is the affinity constant L/mmol, C is the peptide concentration (mmol/L) and mn is the adsorption capacity (µmol/mg of Al). The adsorption capacity mn was 0.086 µmol/mg of Al and the intercept b (2.057 L/mmol) was obtained by solving the monolayer slope. For the second layer, mn was 0.359 µmol/mg of Al, and 0.324 L/mmol for b. If adsorbed molecules in the first organisation are found in condensate state on the solid surface; then it is evident that the amount of retained peptide mn in the second organisation is higher, since adsorbed molecules in this concentration range come into contact with their own condensed phase, which would act as dissolvent in itself. The adsorption coefficient b in the first layer is higher as it measures direct peptide adsorption on the surface; whereas in the second layer, b represents part of the surface interaction, which can transcend adsorbed molecules, as well as the intermolecular interaction between adsorbed peptide, and that forming the double layer.
Such adsorption can be explained by the fact that peptides are complex molecules and their surface retention depends on their physical and chemical properties, but also by the aminoacids' position in the molecular mass sequence and tridimensional structure. Moreover, adsorption on AH is the final result of several molecular interactions and structural arrangements in the adsorbed layer.
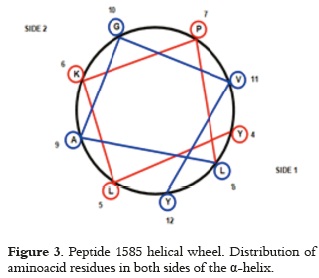
NMR-determined peptide 1585 structure (22) has shown an alpha helix region ranging from aminoacid 4 - 12, and a random aminoacid 12- 20 region in the helical wheel diagram shown in figure 3. It can be observed that most hydrophobic aminoacids are located on side 1; this molecule face will move away from the adsorbing surface since AH is in aqueous media and it does not contribute significantly to peptide adsorption. Adsorption will be considerably affected by charge repulsion, mainly by lysine (K6), when the other side of the helix is orientated towards the adsorbing surface, and because peptide and adsorbent net charges are positive (9.2 peptide 1585 isoelectric point and AH zero charge point 11 at physiological pH). It may thus be considered that if the peptide is orientated towards the surface by the non-structured region (where arginine (R) and lysine aminoacids (K) from positively-charged positions 13, 16 and 17 are found), there is a strong electrostatic repulsion with the surface, adsorption of peptide orientated in this way becoming negligible. Peptide interaction with the adsorbant would only be possible via glutamic acid (E) residues from negatively-charged positions 1 or 20, where an attractive interaction can appear. whose intensity depends on the balance of positive molecule charges and repulsion magnitude generated on the surface.
Equation 2 was used to prove if the adsorbate interaction with the surface occurs simultaneously via the glutamic acid residue of the amino and carboxyterminal ends at two sites on the adsorbent, in the same way as it occurs in dissociative adsorption.

In equation 2, exponent ½ implies two peptide contact points on the adsorbing surface.
The constants were calculated by the linearization of equation 2 thereby generating equation 3.

This adsorption model did not interpret the observed data because the value of the intercept was negative, thereby implying monolayer capacity lacking physical sense.
This result confirmed that adsorption only occurs at one site (preferentially at the peptide amino-terminal end, where a glutamic acid (E1) residue is found) according to a monolayer formation molecular mechanism.
Adsorption model for the first organisation of the adsorbed layer.
The molecular docking (see figure 4a) determined the minimum retention distance of two peptide molecules on the adsorbant surface at nearby sites without attractive or repulsive interactions taking place (i.e. as in the Langmuir adsorption model). It establishes that when a peptide molecule approaches an already adsorbed molecule facing the helical region's hydrophilic sides (due to position 6 lysine), electrostatic and Van der Waals type repulsive interactions are caused with a 4.7 x 106 mV total energy, which decreased to 0 when the molecules were found at 3.7 nm mean distance.
Figure 4b shows that the total energy was 2.1 x 104 mV when lysines were orientated in parallel (one in front and the other behind) and became zero at 3.1 nm. If a molecule approached the furthest a-helix hydrophobic sides faced with lysine residues (K), as shown in figure 4c, Total Energy was 2.6 x 104 mV, and it decreased to zero when the intermolecular distance was 2.2 nm.
If a molecule approached in such a way that the lysine remained orientated in parallel towards the front or in the same direction (but not in parallel), as shown in figure 4d and 4e respectively, the total repulsion energy was 5.8 x 102 and 3.2 x 102 respectively, and it decreased to 0 at 1.8 nm intermolecular distance.
Taking into account this range of possibilities for the molecular adjustment at active adsorbant sites for first layer formation, it was assumed that the most probable interaction between two nearby molecules (A and B) would be the one implying the minimum energy change, and it would be caused at an intermolecular distance of 1.8 nm, orientating lysine side chains in the same direction and around each of them; three nearby molecules would be sited at 1.8 nm orientated in the same way regarding A and B. The adsorbed molecules would be sited at interactive sites separated by 1.8 nm for propagating this layer, keeping the described orientation, and leading to the minimum energy surface, as it is shown in figure 5a.
Adsorption model for the second molecular organisation in the adsorbed layer
The second layer, which was formed by peptide 1585 by being adsorbed on retained molecules, is presented in the isotherm as it can be seen in figure 2. It can be assumed that when peptide molecules approach the adsorbed ones keeping the same orientation that was described for the first layer, a low-energy (-12 mV) interaction occurs (as determined by the docking software) between the carboxy-terminal of the positively-charged retained molecules (due to lysine in positions 16, 17), and the amino-terminal of those molecules forming the second layer as it is shown in figure 5b.
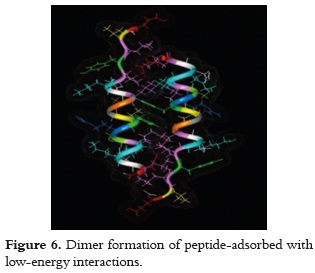
The excess of retained molecules that forms the second layer, regarding those from the first layer, means that others may be set in the interstices of the molecules separated at 1.8 nm. This can be established since the docking simulation with two peptide molecules, which face the helix's hydrophobic side and in an anti-parallel orientation, presented low-energy interactions (-130 mV); which, thereby, enables the formation of dimer-type adsorbed molecular aggregates, as it is shown in figure 6.
The proposed adsorption model for peptide 1585 retention on AH is in agreement with adsorption models known for having ionic tensoactive agents on charged surfaces (24-26).
As a peptide molecule can present areas with different polarity and a rather flexible tridimensional structure, it is possible that strong intermolecular interactions appear, inducing the formation of aggregates that have a certain degree of order as in the already-mentioned models. Some studies have shown evidence of α-helix peptide structure which is usually anphypathic, having some properties associated with tensoactive agents that can form micellar aggregates (26).
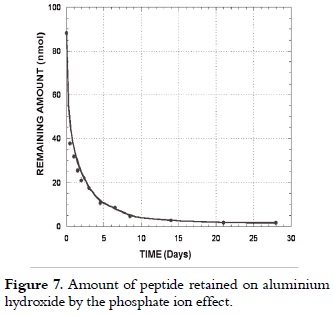
Peptide 1585 release
Release tests, as shown in figure 7, showed that the amount of peptide retained on hydroxide surface decreased remarkably for the first hours, and then it slowly decreased due to the competitive effect of the phosphate ion, preferentially adsorbed on AH, thereby displacing the peptide molecule.
This pattern can be described by the equation 4 (27).

In equation 4, CR is the amount of remaining peptide (nmol), CO is the total amount of adsorbed peptide at 310 K, kL is the release constant (days-1) and t is the time (days).
The following transformation (equation 5) was carried out to corroborate if this equation correctly interpreted peptide 1585 desorption throughout all time ranges, and for determining the constants.

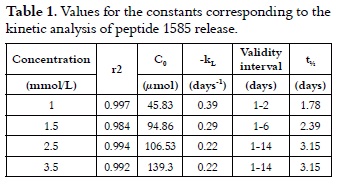
Thus, lnCR = 3.82 – 0.39t, with a correlation coefficient r2 of 0.998, which was obtained by plotting the experimental data according to equation 5, where the total adsorbed concentration at 310 K was 45.83 µmol, and the release constant was 0.39 days-1 representing the peptide 1585 specific desorption rate.
By substituting the value of these constants in equations 4 and 5, the experimental data having dispersion no greater than 1.87% reproduced peptide release until day 2 when 76.27% of the total adsorbed peptide had been released at 310 K from a 1 mmol/L solution. The model did not comply for longer times since the desorbed peptide concentration in the environment remarkably increased the remaining adsorbed amount in an ''in vitro'' system, decreasing the release rate by diffusion effects, and thereby displacing equilibrium.
Results showed that peptide 1585 release at 310 K in the presence of the phosphate ion is a fast process governed by first order kinetics and limited to a two-day diffusion. This fact was not predictable for ''in vivo'' systems where release site may not be present, since the released substrate is processed for presentation to immune system cells.
If the result of adsorption at 298 K is compared to the one obtained at 310 K, then it is concluded that adsorption is affected by temperature, and reiterates the high release found at 310 K. If this result is correlated with the usual procedure, where the peptide is adsorbed at 298 K, conserved at 313 K, and inoculated at 310 K, then a patient will receive an almost instantaneous 57.36% peptide dose; while the controlled release at 310 K will occur for the remaining 42.64% concentration. This might be convenient since it raises the possibility of its recognition by immune system cells, but may cause unwanted allergic reactions.
The same calculations for peptide 1585 release with adsorptions performed with 1.5, 2.5 and 3.5 mmol/L solutions gave a similar pattern, thereby validating the proposed model.
The time taken for the 50% release of the remaining peptide (t½) was calculated by only considering the time range for the peptide 1585 Plasmodium falciparum 190 Trujillo et al release (i.e. the controlled release), thereby generating equation 6.

In equation 6, t½ is the mean life time and kL is the release constant. Table 1 summarizes the results, and it shows that t½ increased with the amount of retained peptide for AH up to 3.15 days.
Adsorption reversibility
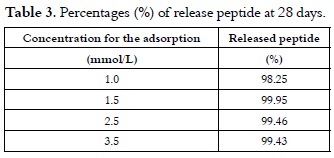
Results presented in table 2 and those illustrated in figure 1, curves (a) solid line and (b) dotted line, showed that the released peptide maintained its physicochemical and structural characteristics before adsorption and after their release. Results shown in table 3 allowed us to infer that peptide 1585 adsorption is totally reversible in the experimental conditions described here.
Peptide 1585 adsorption on AH generates complex isotherms, suggesting the formation of several layers around the adsorbent particles. This can be interpreted by applying Langmuir's model independently to the formation of each layer, while the adsorption coefficient is higher in the first layer and the adsorption capacity is higher in the second. Adsorbed peptide release on AH in the presence of phosphate was performed according to first order kinetics, and it was limited by diffusion effects. Adsorption was totally reversible in the presence of the 4 mM phosphate ion, with pH 7.0 at 310 K, since desorbed molecules showed identical physicochemical characteristics to those of the initial peptide.
CONCLUSIONS
Peptide 1585 adsorption on AH generates complex isotherms, suggesting the formation of several layers around the adsorbent particles. This can be interpreted by applying Langmuir's model independently to the formation of each layer, with the adsorption coefficient being greater in the first layer and adsorption capacity greater in the second.
Adsorbed peptide release on AH in the presence of phosphate was performed according to a 1st order kinetic, which was limited by diffusion effects. Adsorption was totally reversible in the presence of the 4 mM phosphate ion, with pH 7.0 at 310 K, since desorbed molecules showed identical physicochemical characteristics to those of the initial peptide.
Even though the results obtained in this study cannot be extrapolated to an ''in vivo'' vaccination system with peptide molecules, this is the first time that adsorption, release and its reversibility have been physico-chemically characterised. Such parameters have an invaluably importance if they are used for formulating adsorbed peptide vaccines on a frequently used adjuvant like AH. The experience gained in this study draws the attention to the need for standardising physicochemical conditions for optimising the adsorption, which has a paramount importance in formulating a molecule for administering it and presenting it to the immune system.
ACKNOWLEDGMENTS
We would like to thank Universidad Nacional de Colombia for their financial support and Jason Garry for reading the manuscript.
REFERENCES
1. Al-Shakhshir RH, Regnier FE, White JL., Hem SL. Contribution of electrostatic and hydrofobic interactions to the adsorption of proteins by aluminium-containing adjuvants. Vaccine. 1995 Jan; 13 (1): 41-44. [ Links ]
2. Iyer S, Robinett R, HogenEsch H, Hem SL. Mechanism of adsorption of hepatitis B surface antigen by aluminium hydroxide adjuvant. Vaccine. 2004 Mar 29; 22 (11-12): 1475-1479. [ Links ]
3. Wittayanukulluk H. Effect of microenviroment pH of aluminium hydroxide adjuvant on the chemical stability of adsorbed antigen. Vaccine. 2004 Mar 12; 22 (9-10): 1172-1176. [ Links ]
4. Martin A. Physical Pharmacy. 4th ed. Philadelphia, United States: Lea & Febiger; 1993. p. 282-283 [ Links ]
5. Matheis W, Zott A, Schwanig M. The role of the adsorption process for production and control combined adsorbed vaccines. Vaccine. 2002 Oct 12; 20 (1-2): 67-73. [ Links ]
6. Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminium-containing adjuvants and immunopotentiation. Expert Rev Vaccines. 2007 Oct; 6 (5): 685-698. [ Links ]
7. Wolff L, Flemming J, Schmitz R, Gröger K, Goso C, Müller- Goymann C. Forces determining the adsorption of a monoclonal antibody onto an aluminium hydroxide adjuvant: Inf luence of interstitial fluid components. Vaccine. 2009 Mar 13; 27 (12): 1834-1840. [ Links ]
8. Horbett TA, Brash JL (Editors). Proceedings of the A.C.S. Symposium Series on Proteins at Interfaces II. Blomberg E, Claesson PM. New York: A.C.S. Symposium Series; 1995; Chapter 21, volume 602. Proteins at surfaces studies with the surface force technique; p. 296-310. [ Links ]
9. Tleugabulova D, Falcon V, Penton E. Evidence for the denaturation of recombinant hepatitis B surface antigen on aluminium hydroxide gel. J Chromatogr B. 1998 Dec 11; 720 (1-2): 153-163. [ Links ]
10. Norde W, Giacomelli CA. BSA structural changes during homomolecular exchange between the adsorbed and the dissolved states. J of Biotechnology. 2000 May 26; 79 (3): 259-268. [ Links ]
11. Giacomelli CA, Norde W. The adsorption-desorption cycle. Reversibility of the BSA-silica system. J Colloid Interface Sci. 2001 Jan 15; 233 (2): 234-240. [ Links ]
12. Rinella JV, White JL, Hem SL. Effect of anions on model aluminium adjuvant containing vaccines. J Colloid Interface Sci. 1995 Jun 1; 172 (1): 121-130. [ Links ]
13. Seeber SJ, White JL, Hem SL. Solubilization of aluminiumcontaining adjuvants by constituents of interstitial f luid. J Parenteral Sci Tech. 1991 May-Jun; 45 (3): 156-159. [ Links ]
14. Flarend RE, Hem SL, White JL, Elmore D, Suckow M, Rudi AC, et al. In vivo adsorption of aluminium-containing adjuvants using 26 Al. Vaccine. 1997 Aug-Sep; 15 (12-13): 1314-1318. [ Links ]
15. Heimlich JM, Regnier FE, White JL, Hem SL. The in vitro displacement of adsorbed model antigens from aluminiumcontaining adjuvants by interstitial proteins. Vaccine. 1999 Jul 16; 17 (22): 2873-2881. [ Links ]
16. Merrifield RB, Solid phase peptide synthesis I. The Synthesis of a tetrapeptide. J Am Chem Soc. 1963 Jul 20; 85: 2149-2154. [ Links ]
17. Houghten RA. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of Antigen- Antibody interaction at the level of individual amino acids. Proc Natl Acad Sci USA. 1985 Aug; 82 (15): 5131-5135. [ Links ]
18. World Health Organization. Immunological adjuvants. WHO (Technical Report Series). 1976 Jan 1; 595: 6-8. [ Links ]
19. Smith PK, Krohn R, Hermanson A, Mallia K, Gartner F, Provenzano M, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct; 150 (1): 76-85. [ Links ]
20. Wiechelman KJ, Robert DB, Fitzpatrick JD. Investigation of the bicinchoninic acid protein assay: Identification of the groups responsible for colour formation. Anal Biochem. 1988 Nov 15; 175 (1): 231-237. [ Links ]
21. Trujillo M, Valencia J. Peptide solubility, structure and charge position effect on adsorption by aluminium hydroxide. Revista Colombiana de Química. 2006 Jul-Dec; 35 (2): 135-146. [ Links ]
22. Espejo BF, Cubillos M, Salazar LM, Guzmán F, Urquiza M, Ocampo M, et al. Structure, immugenicity, and protectivity relationship for the 1585 malarial peptide and its substitution analogues. Angew Chem Int Ed. 2001 Dec 17; 40 (24): 4654-4657. [ Links ]
23. Sreerama N, Venyaminov SY, Woody R.W., Analysis of protein circular dichroism spectra based on the tertiary structure classification. Anal Biochem. 2001 Dec 15; 299 (2): 271- 274. [ Links ]
24. Bohmer MR, Koopal LK. Adsorption of ionic surfactants on variable-charge surfaces. 2. Molecular architecture and structure of the adsorbed layer. Langmuir. 1992; 8 (11); 2660-2665. [ Links ]
25. Atkin R,Craig VSJ, Wanless EJ, Biggs S. Mechanism of cationic surfactant adsorption at the solid-aqueous interface. Adv Colloid Interfac 2003 May 30; 103 (3): 219-304. [ Links ]
26. Kogan MJ, Dalcol I, Gorostiza P, Lopez-Iglesias C, Pons M, Sanz F, et al. Self-assembly of the amphipahic helix (VHLPPP) 8 a mechanism for zein protein body formation. J Mol Biol. 2001 Oct 5; 312 (5): 907-913. [ Links ]
27. Cid-Cárcamo E. Cinética de disolución de medicamentos, secretaria general de la Organización de los Estados Americanos, OEA, programa regional de desarrollo científico y tecnológico. Washington; 1992. p. 45-55. [ Links ]