Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.18 no.2 Medellín May/Aug. 2011
NATURAL PRODUCTS
ANTIOXIDANT POTENTIAL OF SOME SPECIES OF THE GENUS Bomarea (ALSTROEMERIACEAE)
POTENCIAL ANTIOXIDANTE DE ALGUNAS ESPECIES DEL GÉNERO Bomarea (ALSTROEMERIACEAE)
Fernando A. ALZATE G.1*, Jorge A. GIL Q.2, Nora del S. JIMÉNEZ U.2, Gabriel J. ARANGO A.2, Bernard WENIGER3
1 Instituto de Biología. Universidad de Antioquia. Calle 67 No 53-108. Medellín, Colombia. alzatef@gmail.com.
2 Grupo de Investigación en Sustancias Bioactivas. Facultad de Química Farmacéutica. Universidad de Antioquia. Calle 67 No 53-108. Medellín, Colombia.
3 Faculté Pharmacie. Pharmacognosie et Molécules Naturelles Bioactives. Université L. Pasteur. Strasbourg, France.
Received: 22 April 2010; Accepted: 14 June 2011
ABSTRACT
This work evaluated the antioxidant activity of ethanol extracts from 11 species of the genus Bomarea (Alstroemeriaceae) by means of two in vitro methods. Values of CE50 between 51 and 333 µg/mL were obtained for DPPH the test, and the highest activity levels were found for B. glaucescens, B. setacea, B. pardina and B. euryantha, which presented a similar CE50 or lower than the reference used, silymarin (70.6 ug/mL). Likewise, the TBARS method showed that the maximum inhibition of lipid peroxidation of the linoleic acid was produced by B. hirsuta (malondialdehyde = 0.429 µM), followed by B. bredemeyerana (0.474 µM), B. callejasiana (0.479 µM), B. euryantha (0.489 µM), B. glaberrima (0.497 µM), and B. setacea (0.500 µM). Additionally, the concentration of phenol compounds was evaluated by the Folin-Ciocalteau method, finding that B. setacea presented the highest content of these (159.75 gallic acid equivalents/mg of extract). Bomarea setacea showed the highest antioxidant properties demonstrated by its free-radical scavenging and significant inhibition capacity of the oxidation of linoleic acid.
Key words: Antioxidant, Bomarea, DPPH, Folin-Ciocalteau, plant TBARS.
RESUMEN
En este trabajo se evaluó la actividad antioxidante de extractos etanólicos de 11 especies del género Bomarea (Alstroemeriaceae) por medio de métodos in vitro. Los valores de CE50 obtenidos con la prueba de DPPH se encuentran entre 51 y 333 µg/mL, siendo los valores más altos los obtenidos para B. glaucescens, B. setacea, B. pardina y B. euryantha, los cuales presentaron CE50 similares o inferiores al referente utilizado: silimarina (70,6 µg/mL). Con el método TBARS se encontró que la máxima inhibición de la lipoperoxidación del ácido linoléico, se produce por B. hirsuta (malondialdehido = 0,429 µM), seguida por B. bredemeyerana (0,474 µM), B. callejasiana (0,479 µM), B. euryantha (0,489 µM), B. glaberrima (0,497 µM) y B. setacea (0,500 µM). Se evaluó además el contenido de compuestos fenólicos por el método Folin-Ciocalteau, encontrando que B. setacea presenta el mayor contenido de éstos (159,75 equivalentes de ácido gálico /mg de extracto). La especie B. setacea presenta mayores propiedades antioxidantes, evidenciado esto en su actividad estabilizadora de radicales libres y significativa capacidad de inhibir la oxidación del ácido linoléico.
Palabras clave: antioxidante, Bomarea, DPPH, Folin-Ciocalteau, TBARS.
INTRODUCTION
Living organisms constantly consume oxygen as a natural part of the process of cell energy production. As a consequence of this metabolic activity, highly reactive molecules known as free radicals are produced. These molecules are chemical species derived from oxidative metabolism which have one or more unpaired electrons in their last energy level (1). This reactive oxygen species (ROS), in which we can find superoxide ion, hydroxyl radical, alcoxyl, peroxyl, nitrogen oxide, oxygen peroxide, singlet oxygen and peroxynitrite (2), has shown multiple types of damage at the cellular level (3). The production of these substances can induce oxidative stress (4, 5), generated by an imbalance of free radicals as product of the increase of its production or the decrease in the ability to eliminate them (6). Increased production of free radicals could initiate and promote the progression of some chronic diseases such as cancer, cardiovascular problems, atherosclerosis, inflammation and other (7-9). The reactive oxygen species acts like molecular targets to search biologically active compounds that possess the ability to reduce or inhibit the effects caused by the action of free radicals.
The continuous raise in public concern of the toxic effects that have been generated by some of the commonly synthetic antioxidants used in food preparation and other edible products, increased the need to look for other sources of antioxidant compounds (10). Some plant taxa, such as members of the families Asteraceae, Euphorbiaceae, Lamiaceae, Zingiberaceae, among others, have been frequently included in analyses of the antioxidant activity (11-15). Many studies have produced promising results due to their ability to generate free-radical scavenger substances such as phenolic compounds, carotenoids, vitamins and nitrogenated compounds, all of which are useful as potential sources of antioxidant compounds.
The Alstromeriaceae family, restricted to the Neotropics, includes the genera Alstroemeria and Bomarea (16), the latter being the most diverse with about 110-120 species distributed from Chile to Mexico (17). Bomarea includes lianescent and erect herbs with abundant inflorescences, growing especially in high and middle lands from the Andes region and Central America. Amazonian indigenous communities have used some species of this genus as food (e.g., B. edulis). Additionally, medicinal properties and toxicities are known for species of the genus (e.g. B. salsilla) (18).
Species of the genus Bomarea are common elements of the high Andean forest of Colombia where they grow as showy plants (17). Some species are considered to be endangered due to the human transformation of the Andean forest landscape. Although few uses have been reported for the genus, inflorescences are frequently harvested as ornamental flowers (16).
In this work, we evaluated the antioxidant capacity of ethanol extracts of 11 Bomarea species, constituting the first evaluation of this activity performed to this group of plants. The end goal is to produce more biological information for this genus and to increase the knowledge of its potentialities as antioxidants.
MATERIALS AND METHODS
Plant material of Bomarea species were collected from several localities in Colombia and one Ecuador (B. glaucescens). All specimens were determined by F. Alzate and vouchers were deposited at the Universidad de Antioquia's herbarium (HUA). Species were collected in four fieldworks carried out through Andean forest zones of Colombia and Ecuador. The plant material, consisting of stems and leaves, was dried in an oven at 45ºC with circulating air for 48 h 400 g of powdered dry plant were extracted exhaustively in 3 L of 96% ethanol during 72 h. The obtained extracts were concentrated under vacuum pressure and stored at 4ºC in dark glass.
PHENOLIC CONTENTS
The phenolic content was determined according to the Folin Ciocalteau method previously modified by Londoño et al., 2006 (19). The reaction mix was composed of 750 μL ultrapure water (Milli-Q), 100 μL of extract to be evaluated, 100 μL of sodium carbonate solution (Na2CO3), and Folin Ciocalteu reagent 2N (Sigma® Chemical Co). The mixture was conserved in the dark for one hour after being agitated. The absorbance was determined at 760 nm, in a UV/Vis spectrophotomemeter Varian Cary®. A calibration curve was made with gallic acid (Sigma® Chemical Co) in a concentration range between 10-100 μg/mL. Results were expressed as gallic acid equivalents (μg/mL) per milligram of dried extract (GAE/mg).
FREE-RADICAL SCAVENGING ACTIVITY
A spectrophotometric assay was utilized based on the method proposed by Brand-Williams et al., 1995 (20) and modified later by Jimenez et al., 2005 (21). Extracts of each plant were concentrated (10.000 μg/ mL) and diluted in a 1:2 (v/v) proportion with DPPH ethanol solution (5.07 x 10–5 M) (Sigma® Chemical Co.) The spectrophotometer was utilized to establish the absorbance at 517 nm five minutes after the reaction initiated. Discoloration was compared with the same ethanol and DPPH solution proportion (1:2).
DPPH discoloration percentage was calculated according to the following equation:
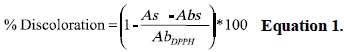
where: As: Sample absorbance, Abs: blank sample absorbance; AbDPPH: blanc DPPH absorbance.
The initial solution was diluted to 75, 50, 25, 10, 5 and 1%, to estimate EC50 for every extract (the necessary concentration to decrease the initial DPPH concentration in 50%). Three tests were made for every extract concentration, ensuring that the variation was not more than 10%. Additionally, Silymarin (Genfar®) 150 mg lot 0509061 was used as a comparison control. Silymarin is a mixture of flavolignanes obtained from Silibus marianus, which is used as a reference in this study because it is a natural free radical scavenger with high antioxidant activity (22).
INHIBITION OF LIPID PEROXIDATION
Previous studies conducted with fully marked antioxidant activity extracts conf irmed the lipoperoxidation inhibition of methyl linoleate to 500 μg/mL (ppm) concentration (23). For this reason, we decided to utilize extracts in a final concentration of 300 μg/mL, defining this concentration limit value as a control parameter to select the most potential antioxidant extracts, for including them in a posterior exhaustive investigation using TBARS methodology. The procedure proposed by Guillensans R, Guzmanchozas, 1998 (24) was modified for this assay. 80 μL of linoleic acid (Sigma® Chemical Co) 20mM, 10μL of each extract (3000 μg/mL) was added by triplicate in the plates. The mixture was pre-incubated and after this stage, 10 μL of CuSO4.5H2O were added. Then, the solution was incubated at 37°C to allow the oxidation of the linoleic acid. After this stage, the oxidation was stopped by means of EDTA addition, and TBA solution was added to the final solution (thiobarbituric acid 0.67% –Sigma Chemical Co, trichloroacetic acid 15% –Merck®, HCl 0.1M – Merck®). The plate was heated at 90°C to allow chromophore formation. After cooling, the content of each plate was filtered and it was read in a Universal Microplate Reader ELx 800Ns at 532 nm.
Results were compared with the positive control, which represents 100% of the lipid matrix oxidation, and finally expressed as μM MDA concentration.
STATISTICAL ANALYSIS
The GraphPad Prism® (Graph- Pad software, Inc, San Diego, CA 2003) statistical package was employed to estimate EC50 and its statistical parameters (goodness of f it and conf idence intervals) in Free radical scavenging activity. An analysis of variance (ANOVA), followed by a Newman–Keuls multiple comparison test, was used to perform a TBARS and Folin Ciocalteau assay. p < 0.05 values were considered significant (*), p < 0.01 very significant (**), and p < 0.001 extremely significant (***).
RESULTS AND DISCUSSION
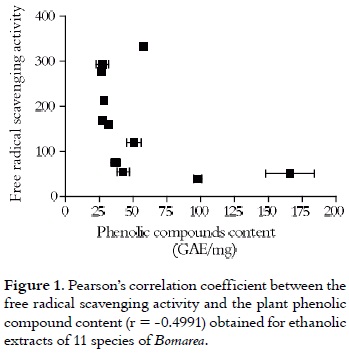
The free radical scavenging activity, the phenolic contents, and the inhibitory effects on linoleic acid peroxidation of ethanolic extracts of 11 Bomarea species are presented in table 1. In general, extracts exhibited a DPPH free radical scavenging activity, which becomes a stable violet radical in ethanolic solution. This radical was absorbed at 517 nm and contains a unpaired electron that can be stabilized, producing a decrease of color, which can be measured by means of an espectrophotometry (25). CE50 varied between 333.3 and 39.0 μg/mL as it is shown in table 1. The extracts of B. glaucescens, B. setacea, B. pardina and B. euryantha showed high free radical scavenger activity, represented by their CE50 value (39.0, 50.99, 54.39 and 75.10 μg/mL, respectively); which is similar to the value found for the reference substance, silymarin (CE50 = 70.66 μg/mL).
The polyphenol content related to the free radical scavenger capacity could indicate that a high content of these compounds constitutes a possible explanation for the behavior observed in the ethanolic extracts of B. glaucescens and B. setacea.
A contrary case is evident for B. pardina and B. euryantha, in which the quantity of phenolic content was relatively low (42.84 ± 4.54 and 37.08 ± 3.61 GAE/mg, respectively), although these had strong free radical scavenger (CE50 = 54.39 and 75.10 μg/ mL, respectively).
The free radical scavenger activity can be attributed to diverse mechanisms that not necessarily involve the plant phenolic content (26). From this point of view, can be re-evaluated as the content of phenolic compounds is a parameter directly correlated with the antioxidant activity, as it was proposed Londoño et al., 2006 (19). The last idea could explain the behavior of the 11 evaluated species, as they show an inverse correlation between polyphenol content and free radical scavenger activity (illustrated in figure 1). This fact was demonstrated by the Pearson's correlation coefficient (r = -0.4991), with r2 = 0.2491, indicating that only 24.91% of the free radical scavenging activity exhibited for these Bomarea species would be explained by the phenolic content in the extracts.
The TBARS test is based on the determination of malondialdehyde (MDA), which is one of the final products of the lipid peroxidation. When this reacts with thiobarbituric acid, it forms a pink chromophore (thiobarbituric acid reactivating substance), which allows to spectrophotometrically follow the reaction to 532 nm (27, 28).
Results (shown in table 1) demonstrated that 50% of extracts present low capacity to inhibit the linoleic acid oxidation, comparatively with the results between the concentrations of MDA (μM) found for the samples, and the positive control of oxidation (100% of the oxidation of the oily matrix). The highest inhibition values of the linoleic acid oxidation were obtained for the B. hirsuta's extract, which had the lowest MDA average concentration (0.429 ± 0.0038 μM) (***p < 0.001).
Extracts of B. setacea (*p < 0.05), B. glaberrima (*p < 0.05), B. euryantha (**p < 0.01), B. callejasiana (**p < 0.01), and B. bredemeyerana (**p < 0.01), presented interesting results to continue the chromatographic isolations studies, because they diminished significantly MDA's formation to a concentration of 300 μg/mL.
The antioxidant activity is often defined by the capacity to delay the beginning of the autoxidation of a substratum for ROS captation, or by the capacity to act like an antioxidant, breaking the chain reaction to disable the phase of the spread of the autoxidation of the mentioned substratum (29). The antioxidant capacity of the extracts of B. setacea, B. glaberrima, B. euryantha, B. callejasiana, and B. bredemeyerana was confirmed by means of the TBARS method, according to their capacity to avoid the formation of malondialdehyde (MDA), which is a product derived from the linoleic acid peroxidation, MDA (26-27).
The response and efficiency to the tests among species, changed according to the method used, reflecting the high complexity of the mechanisms involved in the antioxidant activity, which has been previously postulated by Matkowski et al., 2006 (30). The variability obtained in the response is evident for B. callejasiana, even though representing the extract with low free radical scavenging activity according to the DPPH method (CE50 = 333.3 μg/mL), and having a mean content of phenolic contents (57.56 ± 1.96 GAE/mg). Therefore, it was very effective to disable the linoleic acid peroxidation (MDA μM to 300 μg/mL = 0.479 ± 0.0138**). Similar behaviors appear in the B. glaucescens extract, which presents the highest free radical scavenging activity (CE50 = 39.0 μg/mL), and a great content of phenolic contents (97.89 ± 2.92 GAE/mg). However, it did not show a high capacity to avoid the oxidation of the lipid matrix (MDA μM = 0.532 ± 0.0244*).
The extracts of B. glaucescens and B. setacea presented better stabilizing capacity of free radicals than the reference ones used as control (Silymarin). This fact can be explained by its highest phenol content. The functional groups around the hidroxil aromatic have diverse chemical effects, providing molecules with antioxidant capacities (31).
The results obtained (phenolic content, free radical scavenging and inhibition of lipid peroxidation) strongly support the need for using different methods to establish the antioxidant activity on a complex matrix, such as crude extracts. The antioxidant activity should not possibly be attributed to the presence of specific molecules with phenol content, but it is possibly caused by mutual interactions between the diverse components of the matrix (32). In other studies conducted in parallel to this research, it was possible to determine the presence of phytosterol in Bomarea by means of GC/MS, represented mainly by campesterol, stigmasterol and a great content of β-sitosterol in all the species studied.
The presence of phytosterols might explain the antioxidant potential of several analyzed species, due to the fact that β-sitosterol has shown interesting results regarding its capacity to act as an antioxidant. Nevertheless, results depend on the method used, as it presents a great capacity to stabilize membrane models on which a lipid oxidative process has been induced (33). The assessed extracts showed to have, in addition, a moderate ability to stabilize free radicals (34), which allows considering that the antioxidant capacity is not necessarily involving such mechanism. It is then more likely to propose that this capacity is not due to the mere occurrence of any single metabolite, but rather to the interactions between different components of the analyzed Bomarea's extracts.
Bomarea setacea was the most active extract in this study and a promising taxon with antioxidant activity, which was also evident for three parameters evaluated (CE50 = 50.99 μg/mL, 166.00 ± 17.99 GAE/mg and MDA μM to 300 μg/mL = 0.500 ± 0.0075* for the DPPH, Folin-Ciocalteau and TBARS tests respectively). Future chromatographic isolation is necessary for the extracts of the species with antioxidant activity, such as B. euryantha, B. glaucescens, B. pardina and B. hirsuta, aiming to establish the potential metabolites responsible of the antioxidant response.
CONCLUSIONS
This study constitutes the first evaluation of the antioxidant potential for species of the Bomarea genus, demonstrating that some of the extracts present marked antioxidant activity represented by their capacity to stabilize DPPH free radical, and by their high content of phenolic compound and inhibition of lipid peroxidation. These species can constitute prospective sources of metabolites, useful in the treatment of problems derived from the oxidative metabolism (35-37). The antioxidant activity represents an additional biological activity reported for the genus, which has already been successfully tested in the control of protozoa (38).
ACKNOWLEDGEMENTS
The authors would like to thank Julián Londoño and Edison Osorio for their useful comments and suggestions related to this research, and the Research Group in Bioactives Substances (GISB) of the Universidad de Antioquia (Colombia), project E01467 Sostenibilidad 2009-2010, for supporting this work. The national doctorate program of the Colombian Institute for Science Development (Colciencias) partially supported this project.
REFERENCES
1. Kopani M, Celec P, Danixovi L, Michalka P, Biro C. Oxidative stress and electron spin resonance. Clin Chim Acta. 2006 Feb; 364 (1-2): 61-66. [ Links ]
2. Taylor A, Matalon S, Ward P, eds. Physiology of oxygen radicals. Bethesda, United States: Am Physiol Soc. 1986. Grisham M, McCord J. Chemistry and cytotoxicity of reactive oxygen metabolites. p. 1-18. [ Links ]
3. Flanagan S, Moseleya P, Buettnera G. Increased flux of free radicals in cells subjected to hyperthermia: detection by electron paramagnetic resonance spin trapping. FEBS lett. 1998 Jul; 431 (2): 285-286. [ Links ]
4. Sas K, Robotka H, Toldi J, Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007 Jun; 257 (1-2): 221-239. [ Links ]
5. Kiruthiga P, Shafreen R, Pandian S, Arun S, Govindu S, Devi K. Protective effect of silymarin on erythrocyte haemolysate against benzo(a)pyrene and exogenous reactive oxygen species (H2O2) induced oxidative stress. Chemosphere. 2007 Jul; 68 (8): 1511-1518. [ Links ]
6. Ardestani A, Yazdanparast R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007 Jan; 104 (1): 21-29. [ Links ]
7. Hwang E, Kim G.H. Biomarkers for oxidative stress status of DNA, lipids, and proteins in vitro and in vivo cancer research. Toxicology. 2007 Jan; 229 (1-2): 1-10. [ Links ]
8. Valko M, Leibfritz D, Moncola J, Cronin M, Mazura M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell B. 2007 Jan; 39 (1): 44-84. [ Links ]
9. Montine K, Quinn J, Montine T. Membrane lipid peroxidation. Mattson M eds. Membrane Lipid Signaling in Aging and Age- Related Disease. Amsterdam, Netherland: Elsevier; 2003. p. 11-26. [ Links ]
10. Wang W, Wu N, Zu Y, Fu Y. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008 Jun; 108 (3): 1019-1022. [ Links ]
11. Tepe B. Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia virgata Jacq, Salvia staminea Montbret & Aucher ex Bentham and Salvia verbenaca L. from Turkey. Bioresour Technol. 2008 Apr; 99 (6): 1584-1588. [ Links ]
12. Habsah M, Amran M, Mackeen M, Lajis N, Kikuzaki H, Nakatani N, et al. Screening of Zingiberaceae extracts for antimicrobial and antioxidant activities. J Ethnopharmacol. 2000 Oct; 72 (3): 403-410. [ Links ]
13. Kumaran A, Karunakaran R. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT. 2007 Mar; 40 (2): 344-352. [ Links ]
14. Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sökmen A, Akpulat H. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J Ethnopharmacol. 2003 Aug; 87 (2-3): 215-220. [ Links ]
15. Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M, Bruni R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005 Aug; 91 (4): 621-632. [ Links ]
16. Alzate F. Three new species of Bomarea (Alstroemeriaceae) from the Andean region of Colombia. Novon. 2005 Jul; 15 (2): 253- 258. [ Links ]
17. Alzate F, Mort M, Ramirez M. Phylogenetic analyses of Bomarea (Alstroemeriaceae) based on combined analyses of nrDNA ITS, psbA-trnH, rpoB-trnC and matK sequences. Taxon. 2008 Aug; 57 (3): 1-10. [ Links ]
18. Hofreiter A. Bomarea edulis (Tussac) Herb. a nearly forgotten pre-Columbian cultivated plant and its closest relatives (Alstroemeriaceae). Feddes Repert. 2006 May; 117 (1–2): 85-95. [ Links ]
19. Londoño J, Montoya G, Guerrero K, Aristizabal L., Arango G. Los jugos de cítricos inhiben la oxidación de lipoproteínas de baja densidad: relación entre actividad captadora de radicales libres y movilidad electroforética. Rev Chil Nutr. 2006 Dec; 33 (3): 544-551. [ Links ]
20. Brand-Williams W, Cuvelier M, Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995; 28 (3): 25-30. [ Links ]
21. Jiménez N, Londoño J, Arango G. Actividad captadora de radicales libres y citotoxicidad de plantas colombianas de la familia annonaceae. Lat Am J Pharm. 2005 Mar; 24 (3): 337-42. [ Links ]
22. Dixit N, Baboota S. Kohli K, Ahmad S, Ali J. A review of pharmacological aspects and bioavailability enhancement approaches Silymarin. Indian J Pharmacol. 2007 Aug; 39 (4): 172-179. [ Links ]
23. Kahkonen M, Hopia A, Vuorela H, Rauha J, Pihlaja K, Kujala T. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999 Sep; 47 (10): 3954-3962. [ Links ]
24. Guillen-sans R, Guzmanchozas M. The thiobarbituric acid (TBA) reaction in foods: a review. Crit Rev Food Sci Nutr. 1998 May; 38 (4): 315-330. [ Links ]
25. Antolovich M, Prenzler P, Patsalides E, Mcdonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002 Jan; 127 (1): 183-198. [ Links ]
26. Mantle D, Eddeb F, Pickering A. Comparison of relative antioxidant activities of British medicinal plant species in vitro. J Ethnopharmacol. 2000 Sep; 72 (1-2): 47-51. [ Links ]
27. Chaudhuri S, Banerjee A, Basu K, Sengupta B, Sengupta P. Interaction of f lavonoids with red blood cell membrane lipids and proteins: Antioxidant and antihemolytic effects. Int J Biol Macromol. 2007 Jun; 41 (1): 42-48. [ Links ]
28. Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin Chim Acta. 2007 May; 380 (1-2): 50-58. [ Links ]
29. Masalkar P, Abhang S. Oxidative stress and antioxidant status in patients with alcoholic liver disease. Clin Chim Acta. 2005 May; 355 (1-2): 61-65. [ Links ]
30. Matkowski A, Piotrowska M. Antioxidant and free radical scavenging activities of some medicinal plants from the Lamiaceae. Fitoterapia. 2006 Jul; 77 (5): 346-353. [ Links ]
31. Klein E, Lukes V. DFT/B3LYP study of the substituent effect on the reaction enthalpies of the individual steps of sequential proton loss electron transfer mechanism of phenols antioxidant action: Correlation with phenolic CAO bond length. J Mol Struct. 2007 Nov; 805 (1-3): 153-160. [ Links ]
32. Tuberoso C, Kowalczyk A, Sarritzu E, Cabras P. Determination of antioxidant compounds and antioxidant activity in commercial oil seeds for food use. Food Chem. 2007 Jan; 103 (4): 1494-1501. [ Links ]
33. Mora-Ranjeva M, Charveron M, Fabre B, Milon A, Muller I. Incorporation of phytosterols in human keratinocytes Consequences on UVA-induced lipid peroxidation and calcium ionophore-induced prostaglandin release. Chem Phys Lipids. 2006 Jun; 141 (1-2): 216-224. [ Links ]
34. Renuka R, Arumughan C. Antiradical efficacy of phytochemical extracts from defatted rice bran. Food Chem Toxicol. 2007 Oct; 45 (10): 2014-2021. [ Links ]
35. Wang W, Goodman M. Antioxidant property of dietary phenolic agents in a human LDL oxidation ex vivo model: interaction of protein binding activity. Nutr Res. 1999 Feb; 19 (2): 191-202. [ Links ]
36. Kondo S, Tsuda K, N, Ueda J. Antioxidative activity of apple skin or f lesh extracts associated with fruit development on selected apple cultivars. Sci Hortic. 2002 Dec; 96 (1-4): 177-85. [ Links ]
37. Javanmardi J, Stushnoff C, Locke E, Vivanco J. Antioxidant activity and total phenolic content of Iranian; Ocimum accessions. Food Chem. 2003 Mar; 83 (4): 547-550. [ Links ]
38. Alzate F, Jiménez N, Weniger B, Bastida J, Gimenez A. Antiprotozoal activity of ethanol extracts of some Bomarea species. Pharm Biol. 2008 Sep; 46 (9): 575-78. [ Links ]