Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.18 no.3 Medellín Sept./Dec. 2011
FOODS: SCIENCE, TECHNOLOGY AND ENGINEERING
EVOLUTION OF THE ANTIOXIDANT CAPACITY OF FRANKFURTER SAUSAGE MODEL SYSTEMS WITH ADDED CHERRY EXTRACT (Prunus avium L.) DURING REFRIGERATED STORAGE
EVOLUCIÓN DE LA CAPACIDAD ANTIOXIDANTE DURANTE ALMACENAMIENTO REFRIGERADO DE SISTEMAS MODELO DE SALCHICHAS TIPO FRANKFURT ADICIONADAS CON EXTRACTO DE CEREZA (Prunus avium L.)
Yeni L. ISAZA M.1*; Diego A. RESTREPO M.1; Jairo H. LÓPEZ V.2; Oscar A. OCHOA G.3; Kenneth R. CABRERA T.1
1 Facultad de Ciencias Agropecuarias. Departamento de Ingeniería Agrícola y Alimentos. Universidad Nacional de Colombia, Sede Medellín. Calle 59A No. 63-20. Medellín, Colombia.
2 Instituto de Ciencia y Tecnología de Alimentos, ICTA. Universidad Nacional de Colombia, Sede Bogotá. Carrera 30 No. 45-03. Bogotá, Colombia.
3 Centro de Investigación y Desarrollo Cárnico. Industria de Alimentos Zenú S.A.S. Medellín, Colombia.
4 Facultad de Ciencias. Universidad Nacional de Colombia, Sede Medellín. Calle 59A No. 63-20. Medellín, Colombia.
* Corresponding Author: ylisazam@unal.edu.co.
Received: 30 November 2010
Accepted: 28 September 2011
ABSTRACT
The aim of this work was to establish the antioxidant capacity evolution in a model system of Frankfurter type sausages with added cherry extract through the Folin Ciocalteu, pH differential, FRAP, ABTS, and DPPH methods. Therefore, the total phenol and total anthocyanin contents, the reducing power, and the radical scavenger activity were monitored during a two-month period of storage time in refrigerated conditions (4 ± 1°C). A factorial experimental design was applied with two factors (cherry extract and storage time), and comparative evaluations were made with respect to a product similarly formulated and elaborated without cherry extract but with added sodium ascorbate (0.05%). Results showed no significant difference (p > 0.05) with respect to the total anthocyanin content for any sausage with added cherry extract; while total phenols, reducing capacity and radical captive activity were significantly higher (p < 0.05) in sausages with cherry extract (for the three doses) than in the case of the sausages with no added cherry extract. Moreover, storage time was significant for all cases, because there was a decrease in all the studied variables related to it.
Key words: Free radical, phenols, anthocyanins, storage time, antioxidant.
RESUMEN
Con el objetivo de determinar la evolución de la capacidad antioxidante de sistemas modelo de salchichas Tipo Frankfurt adicionadas con extracto de cereza, se monitoreó el contenido de fenoles totales, antocianinas totales, el poder reductor y la actividad captadora de radicales, empleando los métodos de Folin Ciocalteu, pH diferencial, FRAP, ABTS y DPPH, respectivamente, durante dos meses de almacenamiento a 4 ± 1ºC. Se aplicó un diseño factorial con dos factores (extracto de cereza y tiempo de almacenamiento) y se realizaron evaluaciones comparativas respecto a un producto testigo de igual formulación y proceso, pero sin inclusión del extracto y con presencia de ascorbato de sodio (0,05%). Los resultados mostraron que no existe diferencia significativa (p > 0,05) en el contenido de antocianinas totales para ninguna de las dosis de extracto en las salchichas; mientras que los fenoles totales, el poder reductor y la actividad captadora de radicales fueron significativamente mayores (p < 0,05) en las salchichas con extracto de cereza (para las tres dosis), respecto a las salchichas testigo; además, el tiempo de almacenamiento fue significativo en todos los casos, mostrándose una disminución de todas las variables con el tiempo de almacenamiento.
Palabras claves: radical libre, fenoles, antocianinas, tiempo de almacenamiento, antioxidante.
INTRODUCTION
Due to the fact that the Frankfurter type sausage presents a high fat content in its composition, it is necessary to avoid spoilage related to lipid oxidation through the use of antioxidants during its manufacturing. In the sausage industry, synthetic antioxidants have been commonly included for this purpose; nevertheless, these compounds have been associated with toxicity and negative effects related to human health. This fact has raised the trend of consumers of buying natural products that prevent spoilage and that can be added to meat products as an alternative to replace synthetic compounds. The use of natural products aims to look for the desirable effect on lipid oxidation, by not affecting the quality of the finished product, obtaining health benefits, supporting the prevention of oxidative issues in the organism, and avoiding products resulting from the oxidative reaction, which are widely recognized to be harmful (1-3). Extracts from natural fruits, like cherry, can be used due to their potential antioxidant effect and their rich composition in chemical compounds that include flavonoids such as anthocyanins, flavan-3-ols and flavonols, as well as nonflavonoid compounds, hydroxycinnamic acids and hydroxybenzoic acids (4, 5). It has been reported that these phenolic antioxidants have several positive effects on human health, such as anti-inf lammatory and anti-carcinogenic (6-8); and they are also important for human nutrition (9). The use of these extracts in meat derivatives is related to their antioxidant activity in that matrix (10), and their stability during the processing and storage steps, which can be an indicative of their preservation features against oxidation (11, 12), and of potential health benefits (13). Even though several methods have been used to measure the antioxidant activity of extracts on meat derivatives, only their action in inhibiting the lipid oxidation has been researched (1, 14-22), and no direct method has been performed on them to measure their antioxidant capacity (23). The main objective of this research is to determine the evolution of the antioxidant capacity using the Folin Ciocalteu, pH differential, FRAP, ABTS and DPPH methods on model systems of Frankfurter type sausages with a cherry extract addition during a storage period of 60 days.
MATERIALS AND METHODS
Reagents
Hydrochloric acid, methanol, potassium chloride, Folin Ciocalteu's reagent, sodium carbonate, ferrous ammonium sulphate, potassium persulphate, and phosphoric acid (85% purity) were obtained from Merck® (Darmstadt, Germany). Sodium acetate and potassium phosphate were obtained from Carlo Erba® (Rodano, Italy). The monohydrated gallic acid (3,4,5–trihydroxybenzoic acid monohydrated) and the free DPPH radical (2,2-Diphenyl-1-picrylhydrazyl) were obtained from Alfa Easer® (Massachusetts, United States). ABTS (2,2'-azinobis(3-ethylbenzothiazoline)- 6-sulphonic acid ammonium salt) and TPTZ (2,4,6-Tri-2-pyridyl-s-triazine) were obtained from TCI AMERICA® (Chuo-ku, tokyo). And Trolox (6-hydroxy-2,5,7,8-tetramethylcromane-2- carboxylic acid), which was used as the antioxidant of reference, was obtained from Aldrich® (Munich, Germany).
Raw materials
The mechanically deboned beef meat (16% fat), the pork backfat, and the mechanically deboned chicken meat were obtained from TECNIAGRO® (Medellín, Colombia) and kept under freezing (-18°C) until its posterior use. The cherry extract powder (VEG STABLETM CHERRY 515) highly soluble in water, made up from evaporated cherry juice (Prunus avium L), sugar cane (Saccharum officinarum), and silica (E551) was bought at TECNAS® S.A. (Medellín, Colombia), distributor of NATUREX® INC (New Jersey, United States).
Sausage preparation
Sausages were made under typical manufacturing conditions in a pilot plant. Three levels of cherry extract were used (0.3, 0.4, and 0.5%) in a standard formulation of selected Frankfurter type sausage, according to the Colombian Technical Standard (NTC) 1325 (24); besides, a control product was made with the same formulation and process but without the extract and formulated with sodium ascorbate (0.05%).
The frozen meat, the fat and the mechanically deboned chicken meat were cut into pieces and separately ground in a grinder with an 8 mm disk (Mainca PT 98). Immediately afterwards, the meat and the chicken meat were blended and homogenized in a cutter (Ramon AS 40–20 L), and the rest of the ingredients were slowly added until getting a fine mass without exceeding 11ºC. The cherry extract (for each formulation) and the color were previously dissolved in water at about 0ºC. Then, the mass was stuffed in a cellulose artificial casing with a diameter of 23 mm by means of a Vemag Robby stuffing machine, producing sausages of approximately 40 g. The thermal process was made in a one-car Talsa static smoking house until reaching an internal temperature of 72ºC (in about 8 minutes). The sausages were cooled down for 15- 20 minutes with running water; then, they were hung and taken to a freezing room until reaching an internal temperature of 2 ± 2ºC. The sausages were vacuum packed (6 units per packing) in high barrier films (upper film Cryovac 1.5 Mills, lower film Cryovac 3.5 Mills) by means of a Tiromat Compact 320 packing machine. The processing of the batches of sausages was done independently and in triplicate. The sausages were stored under refrigeration (at 4 ± 1ºC) until their analysis.
Preparation of the aqueous extract of sausage
In all the performed analyses, the hydrophilic fraction of the sausages was used, for which the method proposed by Liu et al., 2009 (25) was taken as a base, although some modifications were performed. A fine mass of the sausages was obtained through the use of a food Kitchen Aid processor; then, 7 g of the mass was weighed, type III water (10 mL) was added, and the mix was stirred (IKA® C – MAG HS4 stirrer) for 20 minutes at room temperature and away from the light. Later, the mixture was vacuum filtered (qualitative filter paper Advantec® No. 2), and the obtained filtered mix was into 25 mL of type III water. The aqueous extract was kept at room temperature in absence of light until its analysis was performed. For the analysis of the ascorbic acid content, the samples were filtered again (startolon polyamide of 0.45 µm, Sartorius Biolab® Products) for injecting them in the HPLC.
Total phenol quantification
The concentration of the total phenols was measured using the Folin Ciocalteu calorimetric method described by Rojas et al., 2008 (26), even though, a calibration curve with concentrated gallic acid between 0 and 16 mg/L (final concentration) was implemented.
Water (300 µL) and sodium carbonate at 20% were added to 200 µL of the aqueous extract of sausage. After left to stand for 5 minutes at rest, a solution of the Folin Ciocalteu's reagent was added at 50%. Then, the mixture was shaken and after 2 hours at room temperature and in absence of light, the absorbance was measured at 760 nm in an spectrophotometer (Thermo Scientific evolution 60). Finally, the results were expressed as mg of gallic acid/100 g of sausage.
Determination of the total anthocyanin content
The total anthocyanin content was determined through the pH differential method described by Giusti and Wrolstad, 2001 (27). Two buffer systems were used: hydrochloric acid/potassium chloride pH 1.0 (0.025 M), and acetic acid/sodium acetate pH 4.5 (0.4 M).
After determining the suitable dilution for the aqueous extract (absorbance between 0.100- 1.200 at 510 nm), the corresponding buffer was added and the absorbance was measured with a spectrophotometer against a blank 510 and 700 nm. The absorbance was determined according to equation 1:
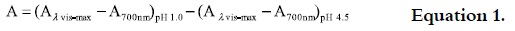
The monomeric anthocyanin concentration (mg/L) was calculated based on the volume of extract and molecular weight, and it was expressed as cianidin 3-glucoside (28), according to equation 2.

when: A = Absorbance; MW = molecular weight of the cianidin 3–glucoside (449.2 g/mol); DF = dilution factor; ε = molar absortivity of the cianidin 3–glucoside (26900 L/mol cm).
The total anthocyanin concentration of the samples was expressed in mg of cianidin 3-glucoside/100 g of sausage.
Quantification of the ascorbic acid content
The quantification was performed through the HPLC technique, according to Gökhmen et al., 2000 (29). For the technique, a Shimadzu prominence 20A series equipment was used, which had one premier column RP-C18 with having the following characteristics: 4.0 mm of diameter, particle size of 5 µm, and a guard column with the same stationary phase of the column. The mobile phase was potassium monobasic phosphate (KH2PO4) adjusted to pH 3 with H3PO4 at 87% at a flow of 1 mL/min. A photodiode array detector was used at 244 nm at an oven temperature of 35ºC. A calibration curve was built with ascorbic acid between 0.01 and 1 mg/mL. The retention time was 4.50 minutes.
Antioxidant capacity
ABTS antioxidant capacity
This process was performed according to the methodology developed by Re et al., 1998 (30) and described by Kuskoski et al., 2005 (28), with a few modifications. The radical ABTS•+ was obtained through the reaction of ABTS with potassium persulphate in buffer phosphate pH 7, incubated at room temperature (± 25ºC) in the dark for 16 h. Once the radical ABTS•+ appeared, it was diluted with buffer phosphate pH 7, until getting an absorbance value of around 0.70 (± 0.1) at 732 nm (wavelength of maximum absorption). The aqueous extract was diluted in type III water until achieving an inhibition percentage between 20 and 80% of the radical in comparison with the absorbance of the blank, after adding a quantity of sample.
The absorbance was determined through the dilution of the radical ABTS•+ at 732 nm and 25ºC. The sample (previously diluted) was added and the absorbance was measured again at 732 nm after 7 minutes. The synthetic antioxidant of reference, Trolox, was tested at concentrations between 0 and 18 µM (final concentration) in methanol, in the same conditions for building the calibration curve. The results were expressed in TEAC o µmol of Trolox/g of sausage (antioxidant activity equivalent to Trolox).
DPPH antioxidant capacity
This determination was performed following the method developed by Brand-Willams et al., 1994 (31), with the modifications described by Kim et al., 2002 (32). The absorbance measuring of the radical DPPH• dissolved in methanol at 80%, was done at a wavelength of 517 nm. The sample was added and the mixture was carefully homogenized; then, it was kept in the dark for 30 minutes. The absorbance measurings at 517 nm were done before the addition of the sample (A0) and after 30 minutes (Af). The synthetic antioxidant of reference Trolox, in concentrations between 0 and 1 mM and diluted in methanol at 80%, was tested under the same conditions for building the calibration curve, thus, expressing the results in TEAC.
FRAP antioxidant capacity
The antioxidant capacity of the sausage aqueous extract was determined by its ability to reduce ferric iron to ferrous iron in a solution of 2, 4, 6–tripyridyl- 2-triazine (TPTZ) prepared in sodium acetate at pH 3.6. The reduction of iron in the TPTZ-ferric chloride solution (FRAP's reagent) led to the appearance of a blue product (tripyridyltriazine ferrous complex). The absorbance of this product was read at 593 nm 4 minutes after the addition of 50 µL of the extract aqueous solution. The standard curve of the antioxidant was developed using ammonium ferrous sulphate. The results were expressed as µmol of Fe2+ equivalents per gram of sausage (33).
Statistical analysis
The analysis of multifactor variance (ANOVA) was performed to the experimental data in order to determine significant effects (p<0.05) of the levels of the cherry extract factor and the storage time factor. Significant differences were determined among the levels of the factors, by contrasts (Tukey Test) among the means. The Statgraphics Centurion XIV software was used to perform the statistical analyses.
RESULTS AND DISCUSSION
Total phenols
The evolution of the total phenol content in time is shown in figure 1. The concentration of these compounds in the Frankfurter type sausage decreased in storage as the days passed, finding significant differences (p<0.05) between the sausages with cherry extract content (0.3, 0.4 y 0.5%) and the control samples, but there was no significant difference among the samples with the three levels of cherry extract addition.
In the freshly cooked sausages, the total phenol content was found to be between 65 and 75 mg of gallic acid /100 g of sausage for the control product and the sausages with the cherry extract, respectively. A higher contribution of phenolic compounds is observed, which is provided by the cherry extract to the sausages, in comparison with the possible contribution provided by the liquid smoke of both formulas (34). After the first 10 days of storage, a drastic decrease of the total phenol concentration was observed, reaching values close to 25 mg of gallic acid/100 g of sausage for the control samples, and values close to 50 mg of gallic acid/100 g of sausage for the samples containing cherry extract. The greater decrease in the total phenol content in the control samples could be due to the compounds provided by the smoke of the formula or the ones naturally present in the sausages, both of which are more unstable than the ones added to the product through the cherry extract. After the first 10 days, the concentration of total phenols kept on decreasing but at a lower rate; this behavior was also observed in other studies with different phenolic compounds (25, 35-37).
Total anthocyanins
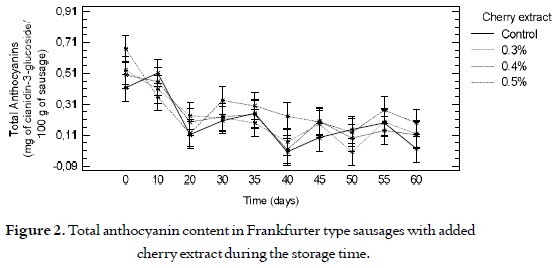
Figure 2 shows a decrease in the total anthocyanin content for all the sausages, expressed as cianidin-3- glucoside in time, finding no significant differences (p > 0.05) among the samples with different levels of addition of the cherry extract and the control samples.
After making the Frankfurter type sausages (day zero), a range between 0.4 and 0.7 mg of Cianidin- 3-glucoside/100 g of sausage was found for the samples from all the cherry extract addition levels. And, at the end of the storage process, values between 0.1 and 0.2 mg of cianidin-3-glucoside/100 g of sausage were found, showing a decrease between 25% and 28% with respect to the initial content. This decrease in the total anthocyanin content can be due to the fact that the compounds are labile and their stability is very variable in function of their structure and the composition of the matrix where they are found (38). Therefore, the compounds are affected by factors such as pH, storage temperature, presence of enzymes, light, oxygen, anthocyanin content, and the presence of other compounds such as flavonoids, proteins and minerals (39).
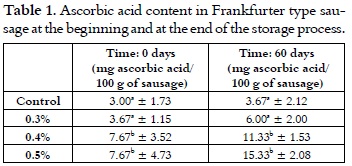
Ascorbic acid
The ascorbic acid content in the freshly cooked sausages goes from 3 mg/100 g of sausage for the control samples, to 7.67 for the samples with the highest cherry extract addition level, as it is shown in table 1. There were no significant difference between the control samples and the sausages with 0.3% of cherry extract addition. But, in both of the performed analyses (day 0 and 60), there were differences between the afore mentioned samples and the sausages with 0.4 and 0.5% of cherry extract in their formula; thus, the higher contribution of ascorbic acid by the cherry extract in these sausages is clearly observable.
Figure 3 shows that the ascorbic acid content at the end of the storage process is higher than it was at the beginning, probably due to its likely generation or recycling (going from dehydroascorbic acid to ascorbic acid) when it is being reduced by enzymes or other substances with the same properties that can be found in the sausages (40, 41).
Antioxidant capacity
ABTS
Figure 4 shows the antioxidant capacity of the ABTS radical of the Frankfurter type sausages, which ranges between 2.8 and 3.1 TEAC/g of sausage and showed a decrease in the storage process as it was observed in the total phenol content. It reached values of 0.8 TEAC/g of sausage for the control product, and 1.9 TEAC/g of sausage for the studied samples with the higher dose of cherry extract (0.5%). These contents represent 28 and 61% of the initial antioxidant capacity for the witness and the sausages with cherry extract, respectively. No significant differences were found (p>0.05) among the sausages with extract concentrations of 0.3% and 0.4%.
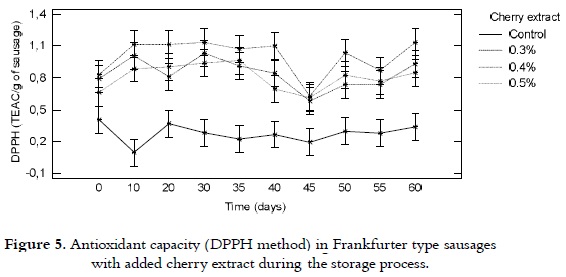
DPPH
The antioxidant capacity, from the point of view of the capacity of scavenging the DPPH radical in the selected Frankfurter type sausages, is shown in figure 5. It was kept relatively constant during the storage process, ranging from 0.7 to 1.2 TEAC/g of sausage for the samples with cherry extract content. While, for the control product, values between 0.3 and 0.4 TEAC/ g of sausage were found. No significant differences among the sausages with intermediate concentrations of the extract (0.3 and 0.4%), while for the extreme concentrations (control samples and samples with 0.5% of extract) there were notable differences.
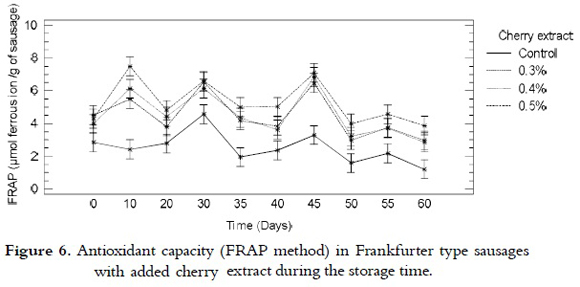
FRAP
For the reducing power analysis that was performed by means of the FRAP method, it was found that there was no significant difference among the treatments with intermediate values of added cherry extract (0.3 and 0.4%), or among the treatments with 0.4 and 0.5% of extract. Nevertheless, there was difference between the samples from all treatments with added cherry extract and the control samples. The reducing power of the iron, expressed as ferrous ion/g of sausage, was relatively constant during the time of storage for all the treatments, as it is shown in figure 6. Even though, in certain days of storage, all the treatments showed a slight increase, possibly due to the regeneration of the compounds responsible of these reactions through the synergistic effects among the antioxidant compounds present in the sausages (42).
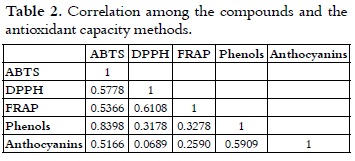
Correlation analysis
Table 2 shows the correlation analysis among the variables: total anthocyanins, total phenols, antioxidant capacity with regard to the ABTS and DPPH radicals, and reducing power (FRAP method).
A high correlation between the ABTS method and the total phenol content (R2 = 0.8398) was found. As a result of this, a decrease in the antioxidant capacity was observed when it was measured by means of the ABTS method at a lower total phenol content. This results were obtained since the ABTS method is based on the transference of hydrogen atoms of the studied antioxidant (43, 44), which is consistent with the way phenols work as a scavenger free radical. This fact occurs because phenols can be donors of hydrogen or electrons in the finishing reactions that break the generation cycle of new free radicals (45). A good correlation was also found between the content of total anthocyanins and total phenols (R2 = 0.5909) as it was expected, due to the fact that anthocyanins belong to the group of phenolic compounds. Moreover, a good correlation (R2 = 0.6108) exists between the DPPH method and the FRAP method, similar to the one reported by Hukkanen et al., 2005 (46), in which values of R2 = 0.675 were found between these two methods. The afore mentioned fact occurs since the FRAP method is based on the transference of electrons (assuming that the antioxidant capacity and the reduction capacity are the same) and, according to Foti et al,. 2004 (47), the DPPH method is based on a reaction of transference of electrons, involving more than one simple atom of hydrogen (43, 47). Thus, it can be observed that the correlation of the FRAP and DPPH methods, with the content of total phenols, shows very similar values: R2 = 0.3278 and R2 = 0.3178, respectively, which correspond to the way phenols react as electron donors. Additionally, in the DPPH and FRAP methods, vitamin C can also be acting since its antioxidant mechanism is based on the transference of electrons to exert its reducing effect, therefore becoming the ascorbate radical (free radical very little reactive); thus, the oxidation propagation phase stops (48-50). Moreover, it has been proved that vitamin C works as a synergistic when it is used in combination with other antioxidants through the promotion or regeneration of the antioxidant properties of other compounds (42).
CONCLUSIONS
The addition of cherry extract to the Frankfurter type sausages provides a high content of compounds with a considerable antioxidant capacity, as it has been proved in comparison with the control product for all the times of storage studied. However, the total phenols, the total anthocyanins and the antioxidant capacity related to the ABTS radical decrease during the storage process, reaching percentages between 25 and 60% of the initial value. The antioxidant capacity related to the DPPH radical and the reducing power were relatively constant during the storage process for all the evaluated treatments. Nevertheless, the values of the sausages with cherry extract were higher than the values of the control sausages in both methods. The content of ascorbic acid at the end of the storage (60 days) showed a slight increase when compared with the initial content, due to the presence of possible regeneration mechanisms of this molecule, among several others in the sausages. The antioxidant capacity (observed in all three methods) in the analyzed sausages is connected to the anthocyanins and phenols content, which suggests that the processing and storage conditions that would help to preserve this compounds in the Frankfurter type sausages should be sought in order to make the possible benefits last until the very moment consumers buy the products.
REFERENCES
1. McCarthy T, Kerry J, Kerry J, Lynch P, Buckley D. Assessment of the antioxidant potential of natural food and plant extracts in fresh and previously frozen pork patties. Meat Sci. 2000 Jul 20; 57 (2): 177-184. [ Links ]
2. Wong S, Peng L, William J. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2005 Nov 2; 99 (4): 775-783. [ Links ]
3. Jimenez F, Carballo J. Healthier meat and meat products: their role as functional foods. Meat Sci. 2001 Jun 6; 59 (1): 5-13. [ Links ]
4. Gao L, Mazza G. Cha racterization, quantif ication, and distribution of anthocyanins and colorless phenolic in sweet cherries. J Agric Food Chem. 1994 Nov 10; 43 (2): 343-346. [ Links ]
5. Gonçalves B, Landbo A, Knudsen D, Silva A, Moutinho J, Rosa E, et al. Effect of Ripeness and Postharvest Storage on the Phenolic Profiles of Cherries (Prunus avium L.). J Agric Food Chem. 2004 Jan 15; 52 (4): 523-530. [ Links ]
6. Garcia R, Gonzalez C, Agudo A, Riboli E. Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Cause Control. 1998 Sep 29; 10 (1): 71-75. [ Links ]
7. Kroon P, Williamson G. Hydroxycinnamates in plants and food: current and future perspective. J Sci Food Agric. 1999 Mar 1; 79 (3): 355-361. [ Links ]
8. Mamani M, Kauss T, Al A, Rambert J, Fawaz F, Thiolat D, et al. Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decrease macrophage inf lammatory mediators. Biochem Pharmacol. 2006 Nov 15; 72 (10): 1304-1310. [ Links ]
9. Usenik V, Fabcic J, Štampar F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008 Mar 1; 107 (1): 185-192. [ Links ]
10. Aruoma O. Assessment of potential prooxidant and antioxidant actions. J Am Oil Chem Soc. 1996 Jul 25; 73(12): 1617-1625. [ Links ]
11. Del Carlo, Sacchetti G, Di Mattia C, Compagnone D, Mastrocola D, Liberatore L, et al. Contribution of the phenolic fraction to the antioxidant activity and oxidative stability of olive oil. J Agric Food Chem. 2004 May 25; 52 (13): 4072-4079. [ Links ]
12. Ninfali P, Bacchiocca M, Biagiotti E, Servili M, Montedoro G. Validation of the oxygen radical absorbance capacity (ORAC) parameter as a new index of quality and stability of virgin olive oil. J Am Oil Chem Soc. 2002 Jun 22; 79 (10): 977-982. [ Links ]
13. Serafini M, Del Rio D. Understanding the association between dietary antioxidants, redox status and disease: Is the Total Antioxidant Capacity the right tool?. Redox Rep. 2004 Mar 28; 9 (3): 145-152. [ Links ]
14. Britt C, Gomaa E, Gray J, Booren A. Influence of Cherry Tissue on Lipid Oxidation and Heterocyclic Aromatic Amine Formation in Ground Beef Patties. J Agric Food Chem. 1998 Nov 7; 46 (12): 4891-4897. [ Links ]
15. Coronado S, Trout G, Dunshea F, Shah N. Antioxidant effects of rosemary extract and whey powder on the oxidative stability of wiener sausages during 10 months frozen storage. Meat Sci. 2002 Jan 6; 62 (2): 217-224. [ Links ]
16. Ahn J, Grün I, Fernando N. Antioxidant properties of natural plant extracts containing polyphenolic compounds in cooked ground beef. J Food Sci. 2002 Jan 9; 67 (4): 1364-1369. [ Links ]
17. Nissen L, Byrne D, Bertelsen G, Skibsted L. The antioxidative activity of plant extracts in cooked pork patties as evaluated by descriptive sensory profiling and chemical analysis. Meat Sci. 2004 Jun 17; 68 (3): 485-495. [ Links ]
18. Sebranek J, Sewalt V, Robbins K, Houser T. Comparison of a natural rosemary extract and BHA/BHT for relative antioxidant effectiveness in pork sausage. Meat Sci. 2004 Sept 18; 69 (2): 289-296. [ Links ]
19. Bozkurt H. Utilization of natural antioxidants: Green tea extract and Thymbra spicata oil in Turkish dry-fermented sausage. Meat Sci. 2006 Feb 28; 73 (3): 442-450. [ Links ]
20. Descalzo A, Sancho A. A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Sci. 2007 Dec 15; 79 (3): 423-436. [ Links ]
21. Nuñez M, Haf ley B, Boleman R, Miller R, Rhee K, Keeton J. Antioxidant properties of plum concentrates and powder in precooked roast beef to reduce lipid oxidation. Meat Sci. 2008 Apr 23; 80 (4): 997-1004. [ Links ]
22. Nieto G, Castillo M, Xiong Y, Álvarez D, Payne F, Garrido M. Antioxidant and emulsifying properties of alcalasehydrolyzed potato proteins in meat emulsions with different fat concentrations. Meat Sci. 2009 Mar 26; 83 (1): 24-30. [ Links ]
23. Sacchetti G, Mattia C, Pittia P, Martino G. Application of a radical scavenging activity test to measure the total antioxidant activity of poultry meat. Meat Sci. 2008 May 7; 80 (4): 1081-1085. [ Links ]
24. Instituto Colombiano de Normas Técnicas y Certif icación. Norma Técnica Colombiana. NTC 1325. Industrias alimentarias. Productos cárnicos procesados no enlatados. Bogotá: ICONTEC; 5ta act; 2008. p. 8, 18. [ Links ]
25. Liu D, Tsau R, Lin y, Jan S, Tan F. Effect of various levels of rosemary or Chinese mahogany on the quality of fresh chicken sausage during refrigerated storage. Food Chem. 2009 Nov 1; 117 (1): 106-113. [ Links ]
26. Rojas D, Narváez E, Restrepo L. Evaluación del contenido de vitamina C, fenoles totales y actividad antioxidante en pulpa de guayaba (psidium guajava l.) de las variedades pera, regional roja y regional blanca [Internet]. Memorias red-alfa lagrotech comunidad europea; 2008 [updated 2010 May 11; cited 2010 Jul 20]. Available from: http://educon.javeriana.edu.co/lagrotech/images/dayana_rojas.pdf. [ Links ]
27. Giusti M, Wrolstad R. Anthocyanins. Characterization and measurement with UV-Visible spectroscopy. Current Protocols in Food Analytical Chemistry. New York: John Wiley & Sons; 2001. p. 2-8. [ Links ]
28. Kuskoski E, Asuero A, Troncoso A, Mancini J, Fett R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Cienc Tecnol Alimentos. 2005 Nov 30; 25 (4): 726-732. [ Links ]
29. Gökhmen V, Kahraman N, Demir N, Acar J. Enzymatically validated liquid chromatographic method for the determination of ascorbic and dehydroascorbic acids in fruit and vegetables. J Chromatogr. 2000 Jun 9; 881 (1-2): 309-316. [ Links ]
30. Re R, Pellegrini N, Proteggente A, Pannala A, Yang X, Rice C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med. 1998 Oct 29; 26 (9-10): 1231-1237. [ Links ]
31. Brand-Williams W, Cuvelier M, Berset C. Use of a Free Radical Method to Evaluate Antioxidant. LWT - Food Sci Technol. 1994Jun 28; 28 (1): 25-30. [ Links ]
32. Kim D, Lee K, Lee H, Yong Lee C. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J Agric Food Chem. 2002 May 22; 50 (13): 3713-3717. [ Links ]
33. Shivashankara K, Isobe S, Al-Haq M, Takenaka M, Shiina, T. Fruit Antioxidant Activity, Ascorbic Acid, Total Phenol, Quercetin, and Carotene of Irwin Mango Fruits Stored at Low Temperature after High Electric Field Pretreatment. J Agric Food Chem. 2004 Feb 10; 50 (5): 893-898. [ Links ]
34. Bortolomeazzi R, Sebastianutto N, Toniolo R, Pizzariello A. Comparative evaluation of the antioxidant capacity of smoke flavouring phenols by crocin bleaching inhibition, DPPH radical scavenging and oxidation potential. Food Chem. 2006 Feb 10; 100 (4): 1481-1489. [ Links ]
35. Chen H, Lin Y, Hsieh C. Evaluation of antioxidant activity of aqueous extract of some selected nutraceutical herbs. Food Chem. 2007 Feb 15; 104 (4): 1418-1424. [ Links ]
36. Yang H, Chang W, Chia Y, Huang C, Lu F, Hsu H, et al. Toona sinensis extracts induces apoptosis via reactive oxygen species in human premyelocytic leukemia cells. Food Chem Toxicol. 2006 Jul 18; 44 (12): 1978-1988. [ Links ]
37 Daood H, Vinkler M, Markus F, Hebshi E, Biacs P. Antioxidant vitamin content of spice red pepper (paprika) as affected by technological and varietal factors. Food Chem. 1995 Jun 23; 55 (4): 365-372. [ Links ]
38. Delgado F, Paredes O. Natural Colorants for Food and Nutraceutical Uses. Florida: CRC Press; 2003. [ Links ]
39. Leyva D. Determinación de antocianinas, fenoles totales y actividad antioxidante en licores y fruto de mora [dissertation]. [Huajuapan de León, México]: Universidad tecnológica de la mixteca; 2009. 80 p. [ Links ]
40. Padayatty S, Katz A, Wang Y, Eck P, Kwon O, Lee J, et al. Review Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J Am Coll Nutr. 2002 May 13; 22 (1): 18-35. [ Links ]
41. Guija H, Troncoso L, Guija E. Propiedades prooxidantes del camu camu (Myrciria dubia). An Fac Med Lima 2005 Dec 10; 66 (4): 261-268. [ Links ]
42. Sánchez A, Djenane D, Torrescano G, Beltrán J, Roncalés P. The effects of ascorbic acid, taurine, carnosine and rosemary powder on colour and lipid stability of beef patties packaged in modified atmosphere. Meat Sci. 2001 May 22; 58 (4): 421-429. [ Links ]
43. Huang D, Ou B, Prior R. The chemistry behind antioxidant capacity assays. J Agric. Food Chem. 2005 Feb 25; 53 (6): 1841-1856. [ Links ]
44. Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free Radical Bio Med. 2010 Aug 15; 49 (4): 503-515. [ Links ]
45. García A. Evaluación in vitro e in vivo de la funcionalidad de un producto rico en antioxidantes [Doctor's thesis]. [Murcia, España]: Universidad de Murcia, España; 2006. 202 p. [ Links ]
46. Hukkanen A, Pölönen S, Kärenlampi S, Kokko H. Antioxidant Capacity and Phenolic Content of Sweet Rowanberries. J Agric Food Chem. 2005 Dec 14; 54 (1): 112-119. [ Links ]
47. Foti M, Daquino C, Geraci C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH radical in alcoholic solutions. J Org Chem. 2004 Feb 27; 69 (7): 2309-2314. [ Links ]
48. Packer L, Traber M, Kraemer K, Frei B, The Antioxidant Vitamins C and E. Illinois, Estados Unidos: AOCS Press; 2002. [ Links ]
49. Asard H, May J, Smirnof f N. Vitamin C Function and biochemistry in animals and plants. New York: Garland Science/BIOS Scientific Publishers; 2002. 191 p. [ Links ]
50. Lebron L. Efecto de diferentes concentraciones de vitamina C (ácido l-ascórbico) en el desarrollo de rancidez oxidativa en filetes de tilapia (oreochromis niloticus) congelados [Master's thesis]. [Mayagüez, Puerto Rico]: Universidad de Puerto Rico. 2006. 10 p. [ Links ]