Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.18 no.3 Medellín Sept./Dec. 2011
BIOTECHNOLOGY
PRODUCTION AND EVALUATION OF ETHANOL FROM COFFEE PROCESSING BY-PRODUCTS
OBTENCIÓN Y EVALUACIÓN DE ETANOL A PARTIR DE LOS SUBPRODUCTOS DEL BENEFICIO DE CAFÉ
Diana P. NAVIA P.1*; Reinaldo de J. VELASCO M.1; José L. HOYOS C.1
1 Departamento de Agroindustria, Universidad del Cauca. Calle 5 No. 4-70. Popayán, Colombia.
* Corresponding autor: dnavia@unicauca.edu.co.
Received: 23 August 2010
Accepted: 29 September 2011
ABSTRACT
Currently, many research studies are being carried out on the production of alcohol from lignocellulosic biomasses. In this study, the ethanol obtained from a mixture of coffee pulp and mucilage, commercial baker's yeast and panela (dehydrated and solidified cane juice) was evaluated. The pulp and mucilage of coffee underwent an acid hydrolysis, and the wort (pulp juice and mucilage) was fermented with an inoculum of exponential phase Saccharomyces cerevisiae and commercial whole panela. The fermented product was distilled, and the gas chromatographic analysis showed an ethanol yield of 25.44 kg/m3, resulting from the 64.40 kg/m3 of total sugars, such yield is equivalent to 77.29% of the theoretical yield. This fact shows that the production of ethanol is viable in small coffee farms using readily available raw materials. The stillage was analyzed and it presented the following values: 0.40 ppm (Iron), 0.97 ppm (Magnesium), 1.54 ppm (Calcium), and 4.40 ppm (Phosphorus). The results confirm that they are particularly useful as a complement in the production of bio-fertilizers for earthworms.
Keywords: Coffee pulp, ethanol, second generation biofuels, Saccharomyces cerevisiae.
RESUMEN
En la actualidad se desarrollan numerosas investigaciones para la producción de alcohol a partir de biomasa lignocelulósica. En este estudio, se evaluó el etanol producido a partir de pulpa y mucílago de café, levadura comercial y panela (jugo de caña solidificado y deshidratado). La pulpa y el mucílago del café se hidrolizaron vía acida y el mosto (jugo de pulpa y mucilago) se fermentó con un inóculo de Saccharomyces cerevisiae en fase exponencial, elaborado con panela entera comercial. El producto fermentado se destiló y su análisis mediante cromatografía de gases arrojó un resultado de 25,44 kg/m3 de etanol a partir de 64,40 kg/m3 de azúcares totales, lo que equivale a un rendimiento del 77,29% respecto al teórico; mostrando que es posible su obtención en pequeñas fincas cafeteras con materias primas de fácil acceso. Se analizaron las vinazas resultantes del proceso, reportando valores de 0,40 ppm (Hierro), 0,97 ppm (Magnesio), 1,54 ppm (Calcio), y 4,40 ppm (Fósforo), lo que las hace particularmente útiles como complemento en el desarrollo de biofertilizantes para la lombricultura.
Palabras clave: pulpa de café, etanol, biocombustibles de segunda generación, Saccharomyces cerevisiae.
INTRODUCTION
The need to seek new alternatives to cover a future energy demand is considerably high nowadays. Moreover, so-called second generation biofuels, particularly bioethanol produced from crop by-products, would most likely be a part of any strategy designed to minimize food security problems (1). Organic waste products, such as mucilage and pulp (2), which are the residue of coffee processing, represent a major source of environmental pollution and their disposal is usually done in the water resources closest to the processing sites, such as rivers and lakes.
Pulp and mucilage consume the oxygen in water, resulting in the death of plants and animals due to the lack of oxygen or the increased acidity (3). This fact can later result in a proliferation of undesirable microorganisms, bringing foul odors, attracting flies and other insects, and rendering the water undrinkable and useless for a many of other uses (4). The idea of using these products came from the need to minimize their negative environmental impacts, to give them added value, and to satisfy the demand for resources suitable for ethanol production. Thus, its use would offer supplies of a second generation liquid fuel wherever coffee is being processed. In turn, this would diversify energy production and promote sustainable development, directly benefiting the inhabitants around rural coffee producing enterprises. This development is facilitated with the implementation of a processing system that uses less water in comparison to the traditional system, and such new system was named Becolsub (ecological coffee processing) by Cenicafe.
A substantial development of biofuels is forecasted in the medium term, and to achieve it, it is necessary to set resources aside for improving the existing technologies, and to research and develop the so-called second generation biofuels at a commercial level. In Colombia, the National Center for Coffee Research (Cenicafé) reports that a method for the production of ethanol from coffee pulp and mucilage is already being developed, and the corresponding feasibility study is also underway (5). The aim of our study is to present a standardized process whereby ethanol can be produced from a mixture of the by-products of coffee processing, integrating the extraction, fermentation and distillation processes of raw materials and evaluating the main process variables.
MATERIALS AND METHODS
Raw materials
The raw materials used were the waste products (mucilage, pulp and juice of the Colombian varietal -Coffea arabica L.) obtained from the ecological coffee processing plant of the ''La Sultana'' plantation owned by the Universidad del Cauca, which is located near the hamlet of Urubamba 2, on the road towards Samboni, in the municipality of Timbío, Cauca Department (Colombia). The sample was collected and stored under refrigeration during its transportation, and it was later processed upon its arrival to the laboratory. Samples of 0.002 m3 were assessed. We used commercial dry baker's yeast Saccharomyces cerevisiae (Levapan, Bogotá, Colombia), and whole panela (Coinprocauca, Popayán, Colombia), which are raw materials readily available in coffee farms. The reagents used in the different methods were all of analytical grade.
Extraction of mucilage and coffee pulp
The coffee beans were harvested fresh, weighed in a balance (Iderna®, Detecto-Colombia, Manizales, Colombia) that complies with the National Federation of Coffee Growers standards. Then, the beans were deposited in a receiving hopper with a capacity of 1,600-1,800 kg, which was then emptied into the pulper. Thanks to the manual operation of a grid that connects the receiving hopper with a small hopper located in the pulper, the pulp was removed from the beans by strain and shear stress. For the correct functioning of the ecological processing plant (a Becolsub 300, equipped with a Gaviota 300 depulper and a DESLIM Demucilager model LIN 300, serial no. 012-2003 manufactured by INGESEC®, Bogotá, Colombia), water was poured into the pulper at a continuous f low rate of 1.53 x 10-5 m3/s.
The pulp extracted from the coffee beans was gathered by hand. This procedure allowed us to obtain information on input and output f lows in the plant, which established the percentage relationship between mucilage and pulp. This percentage relationship was the starting point for the preparation of the wort.
Then, the pulp-free coffee underwent a washing process to remove the mucilage coating the grains (6). A water f low with a rate of 1.67 x 10-5 m3/s was applied to the demucilager. The aqueous mucilage was collected in buckets that were placed just below the plant's output channel. The wet demucilaged coffee was carried through a discharge duct to a concrete storage tank for its subsequent solar drying.
Physicochemical characterization
The determination of moisture content, ash, protein, fat, fiber and carbohydrates was performed using the AOAC method (7).
The determination of acidity was done through the method established in the NMX F-013-SCFI-2000 Standard (8), which is consistent with the Panamerican Standard COPANT 7-3:064. The method is based on the titration of the sample with a standard alkali (0.1 N NaOH) (Carlo Erba, Milano, Italy).
Preparation of wort
The pulp and mucilage were transported to the food laboratory in the Faculty of Agricultural Sciences, University of Cauca, in clean and dry containers and within a portable cooler packed with ice. They were weighed on electronic scales (Javar, JAV-SP, Bogotá, Colombia), tallying with the percentage relationship established in the material weighing. The pulp was arranged in a mesh and pressed to extract as much juice as possible from it without causing physical damage to the structure itself. The mucilage was passed through a mesh to remove some solids, such as coffee parchment or pulp remnants from the wet coffee milling process. The juice obtained from the filtered pulp and mucilage was mixed and transferred to the fermentation site. This wort was subjected to an acid hydrolysis treatment, which consisted in heating it with 37% purity hydrochloric acid (Carlo Erba, Milano, Italy) at 95°C for 900 seconds, at a rate of 6.0 x 10-6 m3 of acid per 1.0 x 10-4 m3 of juice (9).
Fermentation process
Hydration and activation of the yeast
4 g of baker's yeast (Saccharomyces cerevisiae) were weighed and diluted in 4.5x10-5 m3 of sterile distilled water. The solution was stirred until making it homogenous and left to settle for 3 to 8 h at room temperature (10).
Adaptation of inoculum in exponential phase
A solution of whole panela with a concentration of 3% of the total reducing sugars was prepared using sterile distilled water. This solution accounted for 10% of the total volume of wort to be fermented. The solution was heated to 95°C for 900 seconds and rapidly cooled to a temperature of 40°C. The nutrients ammonium phosphate (Carlo Erba, Milano, Italy) (0.14% w/w), urea (Carlo Erba, Milano, Italy) (0.4% w/w), and magnesium sulfate (Carlo Erba, Milano, Italy) (1.1 x 10-5 kg per 1 kg of solution) were added (11). Subsequently, pH was adjusted using sulfuric acid until reaching a value of 4.3; and once the temperature reached 32°C (10-13), the yeast was added according to the proportion of sucrose in the panela solution. This mixture was left to settle for 8 h from the moment of the addition of the yeast. Temperature and pH were kept constant during this period of time.
Fermentation
The wort was hydrolyzed with the yeast inoculation adapted in the exponential phase, and it was subjected to a heat treatment of 95°C for 900 seconds. After reaching a temperature of 40°C, nutrients were added in proportions identical to those of the inoculums. The inoculum obtained in the previous stage was added at pH 4.3 and a temperature of 32°C, starting the fermentation process. At time zero, data on initial density, the concentration of total reducing sugars, and the production of yeast and alcohol were recorded. The temperature and pH values established at the beginning were maintained until the end of the fermentation.
The fermentation was complete when the total reducing sugars in the wort were used up. Another indicator was the reduction to less than four hundredths of the initial density value (11).
Fermentation variables
The fermentation kinetics were evaluated by monitoring the concentration of the substrate (residual sugar), the concentration of the product (ethanol), and the production of yeast. The determination of the total reducing sugar content (ATR) was based on the Lane-Eynon method (9), which is based on the oxidation-reduction Fehling's reaction.
The yeast was determined through a microscope count (Nikon®, Alphaphot s2-H-2, Beijin, China) with a Neubauer camera (BlauBrand, Brand®, Wertheim, Rottweil, Germany) (13, 14).
The density was measured with a hydrometer (Brixco®, Aräometer Dichter nach Klasse H, Germany).
The alcohol content was determined through gas chromatography (GC 14A, Shimadzu®,Kyoto, Japan) (15), using a column for alcohols (BP20, SSG®, Melbourne, Australia). Nitrogen was used as the carrier gas in the column, and hydrogen and dry air were used as the combustion gases in the detector.
Distillation of the product of fermentation
The distillation was performed in a rotary evaporator (Buchi® R-114, Flawil, Switzerland) equipped with a water bath (Buchi® water bath B-480, Flawil, Switzerland), and a vacuum pump (EW-07061-40, Cole-Parmer®, Vernon Hills, Illinois, USA), with an approximate time of 10,800 seconds per batch (2.5 x 10-4 m3) of distillate.
Determination of ethanol concentration through gas chromatography (GC)
Measurements were conducted on a ground and distilled wort sample in a gas chromatograph (GC 14A Shimadzu®, Kioto, Japan) by flame, equipped with an integrator (C-R8A Cromatopac®, Kioto, Japan), using a column for alcohols (BP20, SSG®, Melbourne, Australia). Subsequently, the sample was analyzed by adding anhydrous ethanol. The temperature in the detector and injector was 280 and 100°C respectively.
The temperature vs. time program for the column was set in a range between 45 and 80°C for 360 seconds. The quantification of ethanol using this technique was based on the identification of the analyte retention time (16).
The determination of the ethanol concentration in the samples was performed according to the ''Standard Addition'' method (17).
Analysis of the vinass micronutrients
A atomic absorption spectrophotometry (Solaris AA S5, Thermo Scientific®, Iowa, USA) was performed to determine the contents of calcium, iron, and magnesium, and visible spectrophotometry was performed to determine the phosphorus content.
Atomic Absorption (AA)
All the absorption measurements were performed under the following operation conditions: wave lengths: 213.86 nm for iron, 422.7 nm for calcium, and 285.2 nm for magnesium; hallow cathode lamp current: 11.2 mA; band-pass: 0.5 nm; and integration time: 4 seconds for all elements. The ashes of the stillage sample underwent an acid digestion, using a concentrated hydrochloric acid (Carlo Erba, Milano, Italy). Standard curves were prepared using tritrisol solutions for each metal (Ca, Fe, and Mg) with a concentration of 1000 ppm, from which several solutions were prepared with different concentrations to determine their absorbance in an atomic absorption spectrophotometer (Solaris AA S5, Thermo Scientific®, Iowa, USA). The solution that resulted from the acid digestion was analyzed directly in the atomic absorption spectrophotometer, and the corresponding concentration was calculated based on the previously prepared standard curve. In the case of calcium and magnesium, lanthanum oxide was added to eliminate a possible interference of the matrix (stillage). Lanthanum oxide (Fisher Scientific®, Pittsburgh, USA) was prepared at 0.5% in deionized water, and 1.0 x 10-6 m3 of it was added to the ashes of the stillage sample.
Visible spectrophotometry
The standard curve was prepared from the solutions of anhydrous potassium dihydrogen phosphate (Fisher Scientific®, Pittsburgh, USA). And the absorbance in all solutions was evaluated in a visible spectrophotometer (Milton Roy Company®, Spectronic 20, California, USA).
RESULTS AND DISCUSSION
Extraction of coffee mucilage and pulp
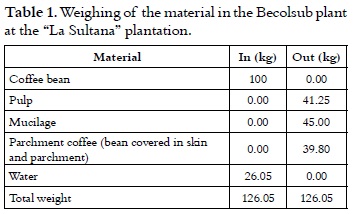
As it is shown in table 1, the weights of the materials show the large amount of water that is added to the process, significantly reducing the soluble solid content that is initially present in the bean from 14 to 8°Brix. This fact represents a major disadvantage in the subsequent fermentation process, requiring the highest possible amount of concentrated sugars in the sample.
Physicochemical characterization
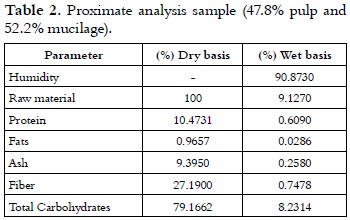
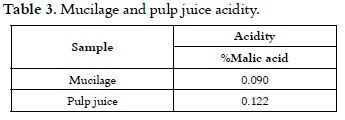
The values in table 2 are similar to those reported for mucilage (9), pulp (18, 19) and pulp mixed with mucilage (20). The results of a proximate analysis and acidity are shown in tables 3 and 4, respectively.
As it has been previously established by other authors (9), it was found that the chemical composition of coffee byproducts changes according to the plantation's height above mean sea level; and at the same time, the composition also changes according to the type of coffee and the stage of development of the fruit when it is harvested. ''La Sultana'' plantation is 1,850 metersabovesea level, which is slightly above the optimum range for growing the Colombian coffee varietal that has been established between 1,200 and 1,800 m (21, 22). Other studies demonstrate the variability in the composition of the different parts of the Colombian coffee varietal fruit when it is planted at different heights (23), the variation of soluble solids according to the maturity (24-27), and the relationship with the processing of coffee (24).
Table 3 presents similar values of acidity within the range of the ones evaluated for the pulp of ripe and overripe coffee fruit (24).
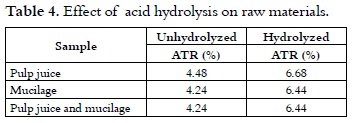
The increase in ATR (total reducing sugar content) that resulted from the acid hydrolysis is similar to the one reported by other authors (9) for mucilage.
After having obtained the raw materials, it was observed that their decomposition occurred immediately after processing.
This decomposition, related to changes in soluble solids, pH, color and odor over time, is associated with factors such as the quantity (table 2) and quality of the water used in the processing of coffee, which was not of drinking quality. The decomposition is also related to the microorganisms present in the raw materials (28, 29), such as Citobacter freundij, Enterobacter cloacae, Geotrichum, and Gram positive cocci (9), which are not only responsible for the immediate fermentation of the mucilage and pulp, but also could compete with the yeast used later in the fermentation, detrimentally affecting the proportions of both substrate and product.
Wort fermentation
The effect of hydrolysis on the juice is presented in table 4. The data showed an increase of more than 50% in the initial content of total reducing sugars for the sample. The acid hydrolysis of polymers, which is not available for the yeast, increases the content of fermentable sugars. According to Ruiz (9), the acid hydrolysis of pectic substances contained in the mucilage, such as protopectin, increases the total sugar content.
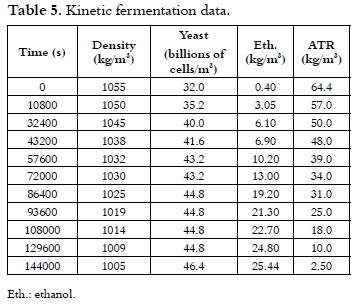
Fermentation variables
Table 5 summarizes the fermentation monitoring data. The fermentation kinetics present a gradual consumption of the available sugars, which are principally related to glucose, fructose and sucrose. A specific growth rate (μ) of 0.018 h-1 was obtained, according to the anaerobic phase of the process.
The exhaustion of the ATR (from 64.4 to 2.5) indicates that fermentation should be stopped at that point in order to avoid a loss of product yield, since the microorganism could possibly take an alternative route for its energy production, finding an available carbon source in the ethanol itself.
This behavior has been reported by other authors (9), where the yeast feeds on the alcohol produced during the fermentation to generate lactic acid, carbon dioxide and water.
On the other hand, it can be observed in the kinetics that the density decreased by five hundredths (from 1055 to 1005), which indicates that the fermentation process was complete (11).
According to Jacques et al., 1999 (12), it is recommended that fermenters are inoculated between 2.0 and 12 billions of cells/m3 for every 1% of substrate in the wort. As it was observed in the fermentation that was carried out with the initial sugar concentration corresponding to 6% w/v, the initial cell content is within the range established in table 5.
Cenicafé obtained 32 kg of alcohol per cubic meter of mucilage from a mechanically operated demucilager using 9.16 x 10-6 m3/s of water (5). In this study, which was carried out in the processing plant of the coffee plantation known as ''La Sultana'' (Cauca, Colombia), pouring 1.53 x 10-5 m3/s of water in the pulp and 1.67 x 10-5 m3/s of water in the demucilager, and fermenting with baker's yeast, 25.44 kg of alcohol were obtained per cubic meter of juice pulp and mucilage. This production is equivalent to 77.29% of the value of alcohol with respect to theoretical yield (30).
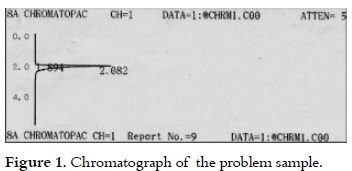
Determination of ethanol concentration through gas chromatography
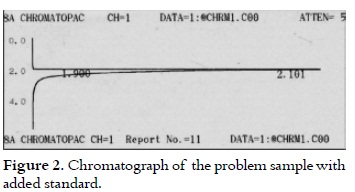
Figure 1 shows the retention time (124.92 s) for ethanol in the sample, which was detected through the gas chromatography. The peak (113.64 s) shown in figure 1 is possibly due to factors such as noise or volatile components that are present in the coffee pulp (31), which retention time was found to be in the range of the temperature and time originally programmed for the column. Similar retention times (126.06 and 114 s) are presented in figure 2, with added standard (anhydrous ethanol), confirming the purity of ethanol in the analyzed samples, which f luctuated in a range between 99.26% and 99.62%.
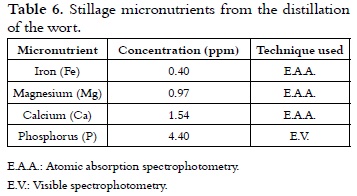
Micronutrient analysis of stillage
Table 6 shows the composition of micronutrients in the stillage obtained as a by-product of the distillation of the fermented samples. This composition is a low mineral content in comparison with the one reported for coffee pulp (32). However, it could be considered as an alternative complement in biofertilizers produced for worms, according to the established nutritional requirements from the place where coffee pulp is used (33).
The following are other uses that these biofertilizers could have: mixes for garden centers, for ornamental plants, for animal feed rations (32) (cautiously, primarily for ruminants), and for the production of edible mushrooms such as Pleurotus ostreatus and Pleurotus sajor, for which mixtures of pulp and mucilage are used (33).
CONCLUSIONS
Ethanol was obtained from wort made from affordable raw materials such as coffee juice and mucilage, baker's yeast and panela. It was necessary to carry out an acid hydrolysis to increase the fermentable sugars content in the wort, and to apply inoculum for efficient performance of the fermentation. This process allows a yield of 77.29% of ethanol with respect to the theoretical value, which can be a viable alternative for obtaining second-generation biofuels in rural areas.
RECOMMENDATIONS
We recommend an evaluation process in a coffee plantation carrying out a cost study.
ACKNOWLEDGMENTS
We would like to thank Colciencias and the University of Cauca for their support in the program for Young Scientists. We would also like to thank John B. Loke of the Ecoenergy Business Group Ltd.
REFERENCES
1. Naik S, Goud V, Rout P, Dalai A. Production of first and second generation biofuels: A comprehensive review. Renew Sust Energ Rev. 2010 Feb; 14 (2): 578-597. [ Links ]
2. García S. Mitigación del impacto ambiental que generan los residuales sólidos del beneficio de café a partir de la producción de abono orgánico [Internet]. Eco-Solar; 2004 Jul [cited 2011 Aug 28]. Available from: http://www.cubasolar.cu/biblioteca/Ecosolar/Ecosolar09/HTML/articulo05.htm. [ Links ]
3. Pandey A, Socool C, Nigam P, Brand D, Mohan R, Roussos S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem Eng J. 2000 Oct; 6 (2): 153-162. [ Links ]
4. Federación Nacional de Cafeteros de Colombia. El café de Colombia, El café: Postcosecha y beneficio [Internet]. Bogotá (CO): La Federación; 2004-2008 [cited 2009 Aug 20]. Available from: http://taran.cafedecolombia.com/caficultura/. [ Links ]
5. Centro Nacional de Investigaciones de Café. Resumen del Informe Anual de Actividades 2008 [Internet]. Chinchiná (CO): Cenicafé; 2008 [cited 2009 Aug 5]. Available from: http://www.cenicafe.org/. [ Links ]
6. Roa G, Oliveros C, Álvarez J, Ramirez C, Sanz J, Dávila A, et al. Beneficio Ecológico del Café. Chinchiná, Colombia: Cenicafé; 1999. 300 p. [ Links ]
7. Association of Official Analytical Chemists AOAC. Official methods of analysis of AOAC International. 16th ed. Gaithersburg, MD: AOAC International; 1995. [ Links ]
8. Secretaría de Comercio y Fomento Industrial. Norma Técnica Mexicana: NMX F-013-SCFI-2000. Café puro tostado, en grano o molido, sin descafeinar o descafeinado, especificaciones y métodos de prueba [Internet]. México D.F.: La Secretaría; 2000 [cited 2009 Jul 29]. Available from: http://www.amcce.org.mx/cafe_puro_tostado.pdf. [ Links ]
9. Ruiz A. Fermentación alcohólica del mucílago del café con levaduras [dissertation]. [Medellín]: Universidad Nacional de Colombia; 1997. 106 p. [ Links ]
10. Hoyos J, Carrera J. Desarrollo de un complejo enzimático por fermentación de sustrato sólido con Rhizopusniveus, para la optimización de la producción de alcohol etílico a partir de melaza. Biotecnol Sect Agropecu Agroind. 2004 Feb; 2 (1): 15-22. [ Links ]
11. Andrade P, Prado O. Manual de Prácticas para el Laboratorio de Operaciones Unitarias III [dissertation]. [Manizales]: Universidad Nacional de Colombia; 2005. 304 p. [ Links ]
12. Jacques K, Lyons T, Kelsall D. The alcohol textbook, A reference for the beverage, fuel and industrial alcohol industries. 3a ed. Nottingham (GB): Nottingham University Press; 1999. 446 p. [ Links ]
13. Gualtieri M, Villalta C, Díaz L, et al. Producción de biomasa de Saccharomyces cerevisiae y Candida utilis usando residuos de pulpa de Coffea arabica L.INHRR. 2007 Dec; 38 (2): 111-130. [ Links ]
14. Reina M. Técnicas de estudio de líneas celulares, técnicas de contaje celular [Internet]. Barcelona (ES): Reina Manuel [updated 2003 Oct 20; cited 2009 Jul 19]. Available from: http://www.ub.es/biocel/wbc/tecnicas/contajecelular.htm. [ Links ]
15. Centro Nacional de Investigaciones de Café. Resumen del Informe Anual de Actividades 2007 [Internet]. Chinchiná (CO): Cenicafé; 2007 [cited 2009 May 2]. Available from: http://www.cenicafe.org. [ Links ]
16. Etxebarria N, Zuloaga O, Olivares M, Bartolomé L, Navarro P. Retention-time locked methods in gas chromatography. J Chromatogr. 2009 Mar; 1216 (10): 1624-1629. [ Links ]
17. Harris D. Análisis Químico Cuantitativo. 3a ed. México: Iberoamérica S.A. de C.V.; 2006. 940 p. [ Links ]
18. Ulloa J, Verreth J, Amato S, Huisman E. Biological treatments affect the chemical composition of coffee pulp. BioresourceTechnol. 2003 Sep; 89 (3): 267-274. [ Links ]
19. Ulloa J, Verreth J, Weerd J, Huisman E. Effect of different chemical treatments on nutritional and antinutritional properties of coffee pulp. Anim Feed Sci Tech. 2002 Aug; 99 (1): 195-204. [ Links ]
20. Blandón G, Dávila M, Rodríguez N. Caracterización microbiológica y físico-química de la pulpa de café sola y con mucílago, en proceso de lombricompostaje. Cenicafé. 1999 Ene-Mar; 50 (1): 5-23. [ Links ]
21. Duque H. Estudio de adopción de la variedad Colombia. Cenicafé. 2005 Apr-Jun; 56 (2): 151-174. [ Links ]
22. Federación Nacional de Cafeteros. El café de Colombia, Caficultura Colombiana: Variedades de café sembradas en Colombia [Internet]. Bogotá (CO): La Federación; 2004-2008 [cited 2009 Aug 20]. Available from: http://taran.cafedecolombia.com/caficultura/. [ Links ]
23. Sadeghian S, Mejía B, Arcila J. Composición elemental de frutos de café y extracción de nutrientes por la cosecha en la zona cafetera de Colombia. Cenicafé. 2006 Oct-Dec; 57 (4): 251-261. [ Links ]
24. Marín S, Arcila J, Montoya E, Oliveros C. Cambios físicos y químicos durante la maduración del fruto de café (Coffea arabica L. var. Colombia). Cenicafé. 2003 Dec; 54 (3): 208-225. [ Links ]
25. Marín S, Arcila J, Montoya E, Oliveros C. Relación entre el estado de madurez del fruto de café y las características de beneficio, rendimiento y calidad de la bebida. Cenicafé. 2003; 54 (4): 297-315. [ Links ]
26. Centro Nacional de Investigaciones de Café. Beneficio Ecológico del Café. Investigación Científica [Internet]. Chinchiná (CO): Cenicafé; 2009 [cited 2009 Aug 3]. Available from: http://www.cenicafe.org. [ Links ]
27. Puerta G. Calidad en taza de algunas mezclas de variedades de café de la especie Coffea arabica L. Cenicafé. 2000 Ener-Mar; 51 (1): 5-19. [ Links ]
28. Do Nascimento L, Do Oiveira L, Picolli R, Fiorini J, Da Silveira S, Schneedorf J, et al. Ozônio e ultra-som: processos alternativos para o tratamento do café despolpado. Cienc Tecnol Alimentos. 2008 Apr-Jun, 28 (2): 282-294. [ Links ]
29. Silva C, Schwan R, Sousa E, Wheals A. Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. Int J Food Microbiol. 2000 Sep; 60: 251-260. [ Links ]
30. Vásquez H, Dacosta O. Fermentación alcohólica: Una opción para la producción de energía renovable a partir de desechos agrícolas. Ing Investig Tecnol. 2007 May; 8 (4): 249-259. [ Links ]
31. Pedroni A, Christen P, Roussos S, Gern J, Soccol C. Coffee residues as substrates for aroma production by Ceratocystis fimbriata in solid state fermentation. Braz J Microbiol. 2003 Sep; 34 (3): 245-248. [ Links ]
32. Barcelos A, De Aguiar P, Olalquialga J, Cardoso R, Dos Santos B. Estimativa das fracoes dos carboidratos, da casca e polpa desidratada de café (Coffea arabica L.) armazenadas em diferentes períodos. Rev Bras Zootecn. 2001 Sep-Oct; 30 (5): 1566-1571. [ Links ]
33. Pineda J. Lombricultura. Tegucigalpa (Honduras): Litografía López; 2006. 38 p. [ Links ]